What is the functional dependence of the 1s orbital energy on Z in Figure 21.7? Check your
Question:
Figure 21.7
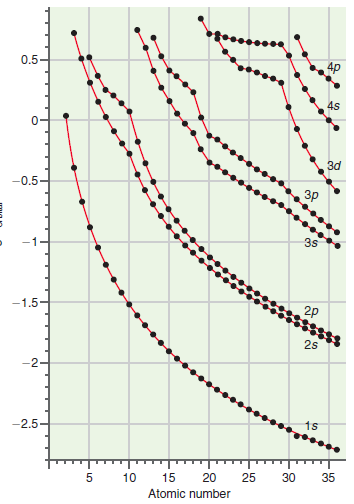
Transcribed Image Text:
0.5 4s Зd -0.5 зр 3s 2p -1.5 2s 15 -2.5 35 30 25 20 10 Atomic number -15
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (12 reviews)
The radial equation for a one electron atom with nuclear charge Z ...View the full answer

Answered By

Sidharth Jain
My name is Sidharth. I completed engineering from National Institute of Technology Durgapur which is one of the top college in India. I am currently working as an Maths Faculty in one of the biggest IITJEE institute in India. Due to my passion in teaching and Maths, I came to this field. I've been teaching for almost 3 years.
Apart from it I also worked as an Expert Answerer on Chegg.com. I have many clients from USA to whom I teach online and help them in their assignments. I worked on many online classes on mymathlab and webassign. I guarantee for grade 'A'.
4.90+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Electron and hole concentrations increase with temperature. For pure silicon, suitable expressions are h = e = 6200T 1.5 e 7000 /T C/m 3 . The functional dependence of the mobilities on temperature...
-
Extend the considerations of the preceding problem to particle diffusion, and assume that there is a net particle generation rate gu that is proportional to the local particle concentration, gu =...
-
Vortex flow (a) Show that the complex potential w = (iT/2) in z describes the flow in a vortex. Verify that the tangential velocity is given by v = T/2 and that v r = 0. This type of flow is...
-
Brennan Physiotherapies had a beta of 0.85. Reasonable estimates for the RF and the required rate of return on the market, R(Rm) were 7% and 15%, respectively. What is the required rate of return on...
-
A privately owned yacht leaves a dock in Myrtle Beach, South Carolina, and heads toward Freeport in the Bahamas at a bearing of S 1.4 E. The yacht averages a speed of 20 knots over the...
-
Problems 1221. The purpose of these problems is to keep the material fresh in your mind so that you are better prepared for later sections, a final exam, or subsequent courses such as calculus. Find...
-
Calculate r2 for the least squares line in Exercise 11.18 (p. 625). LO9
-
You are to construct a star schema for Simplified Automobile Insurance Company (see Kimball, 1996b, for a more realistic example). The relevant dimensions, dimension attributes, and dimension sizes...
-
Record direct materials costs for Jobs 402 and 404. Record direct labor costs for Jobs 402 and 404. Record the entry to allocate overhead to Jobs 402 and 404 at 200% of direct labor costs assigned....
-
7.16 Figure 7.21 shows a pump delivering 840 L/min of crude oil (sg = 0.85) from an underground storage drum to the first stage of a processing system. (a) If the total energy loss in the system is...
-
The total energy eigenvalues for the hydrogen atom are given by E n = e 2 / (8Ïε 0 a 0 n), n = 1, 2, 3, 4,¦, and the three quantum numbers associated with the total energy...
-
Why is the total energy of a many-electron atom not equal to the sum of the orbital energies for each electron?
-
Under the syiterh, entries to record the purchase of merchan dise are recorded in the Merchandise Inventory account.
-
Star Trek LLC has 8,000 bonds, two million shares of preferred stock outstanding and seven million shares of common stock outstanding. If the common shares are selling for $17 per share, the...
-
So the component of the flow velocity that is perpendicular to the isobar in cm/s is: V =2V cos(0)=2(10/(1+2 sin(0))) cos(0) V (10)/[1+2(1) sin(90)] = V = 3.3cm/s
-
2. The CIBC stock price was very volatile today. a. Using the website below, what did the price of CIBC shares end the day at ? CM.TO: Canadian Imperial Bank of Commerce - Yahoo Finance b. In a brief...
-
Answer the following according to Florida rules: Abigail Atlas was chatting quietly in the hall outside Courtroom 14-1 with Mariel Topher, an employee of the Hopper Law Firm that was representing...
-
Draw Free body diagrams for the following 32 situations and write the appropriate x- and y- equations, using the diagram and title to help you (do not solve). Some of the material you have not...
-
In Exercises 3748, determine whether each function is even, odd, or neither. Then determine whether the functions graph is symmetric with respect to the y-axis, the origin, or neither. f(x)=x1-x
-
6 (a) Briefly develop a mathematical model of the behaviour of a copper-twisted pair cable (b) Derive the magnetic energy from: w given that: K + w, where the - - k symbols have their usual meaning...
-
Calculate the percentage change in K x for the reaction H 2 CO(g) CO(g) + H 2 (g) when the total pressure is increased from 1.0 bar to 2.0 bar at constant temperature.
-
A sealed container was filled with 0.300 mol H 2 (g), 0.400 mol I 2 (g), and 0.200 mol HI(g) at 870 K and total pressure 1.00 bar. Calculate the amounts of the components in the mixture at...
-
The equilibrium constant for the gas-phase isomerization of borneol (C 10 H 17 OH) to isoborneol at 503 K is 0.106. A mixture consisting of 7.50 g of borneol and 14.0 g of isoborneol in a container...
-
(15 points) Stressed $2.500,000 of S% 20 year bands. These bonds were issued Jary 1, 2017 and pay interest annually on each January 1. The bonds yield 3% and was issued at $325 8S! Required (2)...
-
Packaging Solutions Corporation manufactures and sells a wide variety of packaging products. Performance reports are prepared monthly for each department. The planning budget and flexible budget for...
-
1. A company issued 10%, 10-year bonds with a par value of $1,000,000 on January 1, at a selling price of $885,295 when the annual market interest rate was 12%. The company uses the effective...

Study smarter with the SolutionInn App


