Why is it useful to define the chemical shift relative to a reference compound as follows? 8
Question:
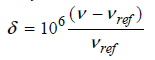
Transcribed Image Text:
8 = 10°- Vref) Vref
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (10 reviews)
The nuclear spin splitting depends on the static magnetic field Thus measuring the same samp...View the full answer

Answered By

Shehar bano
I have collective experience of more than 7 years in education. my area of specialization includes economics, business, marketing and accounting. During my study period I remained engaged with a business school as a visiting faculty member and did a lot of business research. I am also tutoring and mentoring number of international students and professionals online for the last 7 years.
5.00+
4+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
What is a motif? Why is it useful for computer programs to identify functional motifs within amino acid sequences?
-
What is Stirlings approximation? Why is it useful? When is it applicable?
-
The chemical shifts of nitromethane, dinitromethane, and trinitromethane are at 6.10 4.33, 7.52 Match each chemical shift with the compound. Explain how chemical shift correlates with pKa.
-
On November 1, 2021, Sadie's borrows $230,000 from a local bank and signs a note. The note requires interest to be paid annually beginning on August 1, 2022 at 12%. Principal and interest are due in...
-
(a) solve for P and (b) solve for t. A = Pert
-
The article So Close, Yet So Far: Predictors of Attrition in College Seniors (Journal of College Student Development [1998]: 343348) examined the reasons that college seniors leave their college...
-
5 What is the main result of each of the three phases of the strategic marketing process? (a) planning, (b) implementation, and (c) evaluation.
-
Yusane Bolts, Inc., produces 50,000 units each day, and the average number of units in work in process is 200,000. The average annual inventory carrying cost percentage is 25%, and the average work...
-
Northwood Company manufactures basketballs. The company has a ball that sells for $25. At present, the ball is manufactured in a small plant that relles heavily on direct labor workers. Thus,...
-
Create the initial ER diagram for a car dealership. The dealership sells both new and used cars, and it operates a service facility. Base your design on the following business rules: a. A salesperson...
-
Why do neighboring groups lead to a net induced magnetic field at a given spin in a molecule in the solid state, but not for the same molecule in solution?
-
What is the advantage of a 2-D NMR experiment over a 1-D NMR experiment?
-
A therapist tells a 74-kg patient with a broken leg that he must have his leg in a cast suspended horizontally. For minimum discomfort, the leg should be supported by a vertical strap attached at the...
-
Prepare the entries to record the transaction 2 A company has three employees, each of whom has been employed since January 1 earns $2750 per month and is paid on the last day of each month On March...
-
Pet Emporium had a robbery on the weekend in which a large amount of inventory was taken. The loss is covered completely by insurance. A physical inventory count determined that the cost of the...
-
In a test taken by a class of 50 students, the average was 1500 with a standard deviation of 40. What 2 scores capture the middle 60% of the students?
-
For questions 1-8, let P = (-2, 5) and Q = (4,8). 1. Find the distance from the point P to the point Q. 2. Find the midpoint of the line segment joining P and Q. 3. Find the slope of the line PQ. 4....
-
True/False Indicate whether the statement is true or false. ____ 1. In accounting, to value means to record a transaction or event. ____ 2. The recognition issue deals with when a business...
-
Consider the following equations. Solving the second equation for x requires the same logic as solving the first equation for x. Solve the equation. Give the solution set. First Equation 7x + 8 3 =...
-
For the vector whose polar components are (Vr = 1, Vθ = 0), compute in polars all components of the second covariant derivative Vα;μ;ν. To find...
-
What feature of molecular orbital theory is responsible for bond formation?
-
Draw scale vector diagrams to represent the states (i) l=1, m l =+1, (ii) l=2, m l =0.
-
Use the data in Exercise 8C.4(a) to calculate the energy needed to excite a CH 4 molecule from a state with l=1 to a state with l=2. Data in Exercise 8C.4(a) The moment of inertia of a CH 4 molecule...
-
A stock is expected to pay a dividend of $1.50 at the end of the year (i.e., D 1 = $1.50), and it should continue to grow at a constant rate of 10% a year. If its required return is 14%, what is the...
-
The Hobby Shop has a checking account with a ledger balance of $1,700. The firm has $2,400 in uncollected deposits and $4,200 in outstanding checks. What is the amount of the disbursement float on...
-
An investment will pay you $34,000 in 11 years. If the appropriate discount rate is 6.1 percent compounded daily, what is the present value? (Use 365 days a year. Do not round intermediate...

Study smarter with the SolutionInn App


