Question: Draw a first-law bar chart (see Figure 19.12) for the gas process in Figure EX19.6. Figure 19.12 FIGURE EX19.6 (a) Isothermal process: AEh = 0
Draw a first-law bar chart (see Figure 19.12) for the gas process in Figure EX19.6.
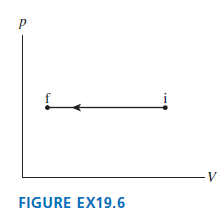
Figure 19.12
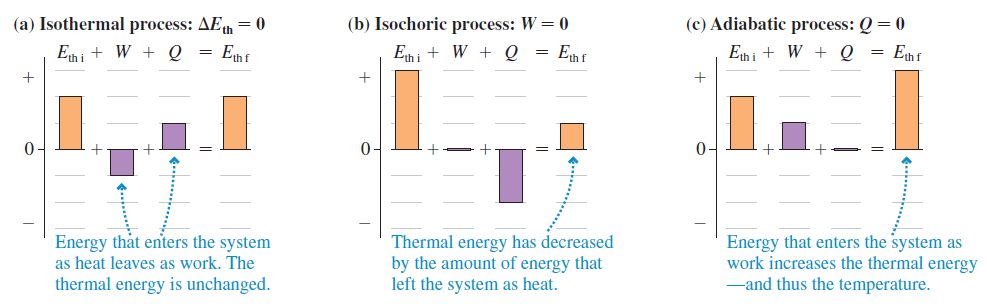
FIGURE EX19.6 (a) Isothermal process: AEh = 0 (b) Isochoric process: W = 0 (c) Adiabatic process: Q = 0 Eth i + W + Q = Enf En i + W + Q Enf Ehi + W + Q = Enf 0- Energy that enters the system as heat leaves as work. The thermal energy is unchanged. Thermal energy has decreased by the amount of energy that Energy that enters the system as work increases the thermal energy -and thus the temperature. left the system as heat.
Step by Step Solution
3.26 Rating (158 Votes )
There are 3 Steps involved in it
Visualize Solve Because this is an isobaric process W pdV pV f V i Since V f ... View full answer

Get step-by-step solutions from verified subject matter experts


