According to Table 1, the concentrations of which of the following substances varies the least with temperature?
Question:
According to Table 1, the concentrations of which of the following substances varies the least with temperature?
A solute is any substance that is dissolved in another substance, which is called the solvent. A student tested the solubility (a measure of how much solute will dissolve into the solvent) of six different substances. The solubility of a substance at a given temperature is defined as the concentration of the dissolved solute that is in equilibrium with the solvent. Table 1 represents the concentration of dissolved substances in 100 grams of water at various temperatures. The concentrations are expressed in grams of solute per 100 grams of water.
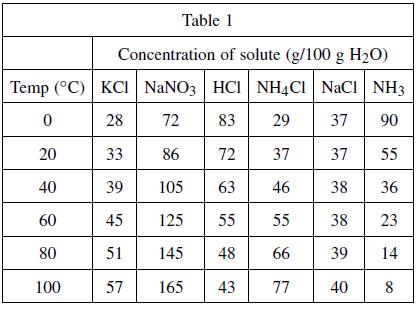
A. HCl
B. NH3
C. NaCl
D. KCl
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





