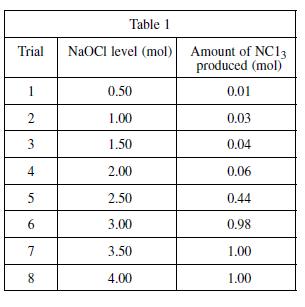
In Experiment 2, different quantities of NaOCl were added to the ammonia solution resulting in the production
Question:
In Experiment 2, different quantities of NaOCl were added to the ammonia solution resulting in the production of nitrogen trichloride. The amounts of nitrogen trichloride produced for 3.00, 3.50, and 4.00 mol of NaOCl added were approximately the same. Which of the following best explains why the production of NCl3 was limited, based on this observation and the results of the experiment?
F. NaOCl absorbs the extra NH3.
G. The amount of chlorine gas produced slows the reaction.
H. NaOCl binds with the H2O in the solution and causes the reaction rate to decrease.
J. The amount of NH3 available for reaction is limited, and once used up, the reaction stops.
Experiment 2
Another known by-product of the reaction of bleach and ammonia is nitrogen trichloride (NCl3). Nitrogen trichloride is a yellow, oily, pungent-smelling liquid, often found as a by-product of chemical reactions between nitrogencontaining compounds and chlorine. It is highly explosive. To determine the quantities of bleach and ammonia that, when mixed together, produce NCl3, again a varying quantity of bleach was added to eight different ammonia–water solutions and the resulting NCl3 from each mixture was measured. A solution of 1.0 mole (mol) of NH3 in 1 kg of water was used in each trial. A certain quantity of NaOCl was added to each solution; the quantity added was gradually increased for each trial. The amount of nitrogen trichloride produced in each trial was recorded in see Table 1.
Step by Step Answer:






