Question
In the presence of sodium ethoxide (NaOCH2CH3), the alkyl bromide below undergoes a concerted elimination to form a trisubstituted alkene. a mechanism with curved
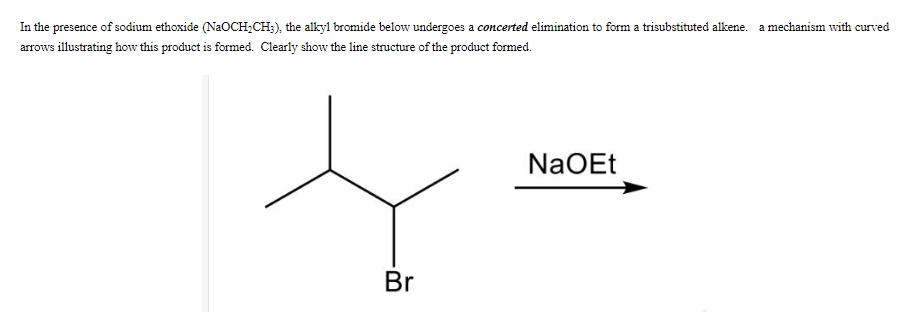
In the presence of sodium ethoxide (NaOCH2CH3), the alkyl bromide below undergoes a concerted elimination to form a trisubstituted alkene. a mechanism with curved arrows illustrating how this product is formed. Clearly show the line structure of the product formed. Br NaOEt
Step by Step Solution
There are 3 Steps involved in it
Step: 1
First the sodium ethoxide NaOEt will act as a strong base and ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Focus On Personal Finance
Authors: Jack R. Kapoor, Les R. Dlabay Professor, Robert J. Hughes, Melissa Hart
5th Edition
0077861744, 978-0077861742
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



