Referring to Figure 9.24, where f 3 / R = 0.5, what behavior would you expect the
Question:
Referring to Figure 9.24, where f3 / R = 0.5, what behavior would you expect the curves to exhibit if f3 / R = 1? Why?
Figure 9.24
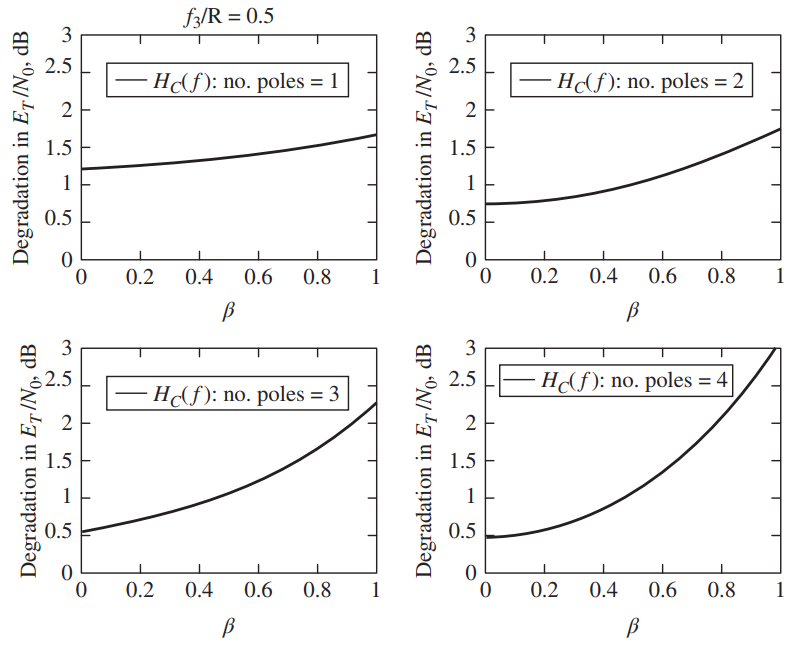
Transcribed Image Text:
f3/R = 0.5 %3D 3 3 2.5 2.5 HAf): no. poles = 1 HAf): no. poles = 2 2 2 1.5 1.5 1 1 0.5 0.5 0.6 0.2 0.2 0.4 0.8 0.4 0.6 0.8 3 2.5 HAf): no. poles = 4 2.5 f): no. poles = 3 2 1.5 1.5 1 0.5 0.5 0.2 0.4 0.6 0.8 0.2 0.4 0.6 0.8 1 Degradation in ErINo, dB Degradation in Er INg, dB Degradation in Er/No, dB Degradation in E7/No, dB 3.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 36% (11 reviews)
If f3R 1 then the curves would exhibit a wider range ...View the full answer

Answered By

Tamondong Riza
Professionally, I am a teacher with years of experience tutoring math and science, as well as teaching in both public schools and independent schools. I feel that education should be an enlightening experience for all children, and I'm committed to helping my students learn new skills and make progress in their subjects.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Principles of Communications Systems, Modulation and Noise
ISBN: 978-8126556793
7th edition
Authors: Rodger E. Ziemer, William H. Tranter
Question Posted:
Students also viewed these Physics questions
-
Find the center of mass of a water molecule, referring to FIGURE 9-43 for the relevant angles and distances. The mass of a hydrogen atom is 1.0 u, and the mass of an oxygen atom is 16 u, where u is...
-
Referring to Figure 20.6, would you expect H2O and H1 to be reduced at the cathode and H2O oxidized at the anode? Battery Impure copper anode Pure copper cathode 2+ So
-
Referring to Figure 2.4 (c), if the height of water (at 4°C) above point 7 is 52.3 cm, what is the height of the oil (sp. g r. = 0.85) above point 8? Oil --@-t - _ NES 10
-
Implement the method contains() for BST.
-
The National Leukemia Society maintains a portfolio of temporary equity investments. At December 31,2013, the Society holds the following securities: Quarterly dividends paid in 2014 on each...
-
If merchandise inventory is being valued at cost and the price level is steadily rising, which of the three methods of costing^fifo, lifo, or average cost^^vill yield (a) the highest inventory cost,...
-
Review the nature of change, the traditional model of planned change and the limitations of this model? L01
-
Rod Manufacturing Company produces metal rods for their customers. Its wholesale division is the focus of our analysis. Management of the company wishes to analyze the profitability of the three key...
-
Problem 3-31 (LO. 4) Analyze each of the characteristics in considering the indicated test for dependency as a qualifying child or qualifying relative. In the last two columns, after each listed test...
-
On March 1 , Kerr Corporation issued 1 0 , 0 0 0 preferred shares for $ 1 0 0 per share. On July 1 5 , it issued an additional 3 0 , 0 0 0 shares for $ 1 2 0 per share. Each share is convertible into...
-
Antipodal base band PAM is used to transmit data through a low pass channel of bandwidth 10 kHz with AWGN background. Give the required value of M, to the next higher power of 2, for the following...
-
What might be an effective communication scheme in a flat-fading channel if one could determine when the channel goes into a deep fade? What is the downside of this scheme? (What would happen if the...
-
Problems 94103 are based on material learned earlier in the course. The purpose of these problems is to keep the material fresh in your mind so that you are better prepared for the final exam. Solve...
-
Prove that Eq. (19.34) gives the simplest multi-gluon and gluon-quark states that contain an \(\mathrm{SU}(3)\) color singlet in the decomposition. Data from Eq. 19.34 (GG)1: (88)1 (Gqq) : [8 (383)8]...
-
In question 70, what is the probability that of the 100 cars test-driven, more than 35 cars get more than 45 miles per gallon? How many of the 100 cars tested would you expect to get more than 45...
-
Construct the braid group products (a) (b) using the algorithm of Fig. 29.16 . Data from Fig. 29.16
-
Worksheet The adjusted trial balance columns of a worksheet for Bond Corporation are shown below. The worksheet is prepared for the year ended December 31. Complete the worksheet by (a) entering the...
-
The Healthy Catering Service had the following transactions in July, its first month of operations: 1 Kelly Foster contributed \(\$ 18,000\) of personal funds to the business in exchange for common...
-
Refer to figure 31.18 and the chapter content to answer the following questions. 1. Using the Survey the Landscape figure in the chapter introduction and figure 31.18, describe how the respiratory...
-
In exchange for land, the company received a 12-month note on January 1. The face amount of the note is $1,000, and the stated rate of interest is 13%, compounded annually. The 13% rate is equal to...
-
Sketch the single-sided and double-sided amplitude and phase spectra of (a) x 1 (t) = 5 cos (12t - /6) (b) x 2 (t) = 3 sin(12t) + 4 cos (16t) (c) x 3 (t) = 4 cos (8t) cos (12t) (d) x 4 (t) = 8 sin...
-
The sum of two or more sinusoids may or may not be periodic depending on the relationship of their separate frequencies. For the sum of two sinusoids, let the frequencies of the individual terms be f...
-
A signal has the double-sided amplitude and phase spectra shown in Figure 2.33. Write a time-domain expression for the signal. |Phase |Amplitude 4 -4 -2 4 4
-
Suppose an investment is equally likely to have a 42% return or a -20% return. The total volatility of returns is closest to: Select one: a. 9.61% b. 43.84% c. 21.92% d. 31.00%
-
Project DEF Initial End-of-Year Investment Cash Flows for years 1-3, respectively $32,000 $20,000 30,000 17,000 WACC = 17% What is the Profitability Index? (Please round to the nearest hundredth and...
-
A company owes $100 to be paid at times 2, 4, and 6. The company plans to meet the obligation with an investment program that produces asset cash flows of A1 at time 1 and A5 at time 5 using...

Study smarter with the SolutionInn App


