Lets assume that you have GBP100 to invest, and have two strategies from which to choose with
Question:
Let’s assume that you have GBP100 to invest, and have two strategies from which to choose with the following cash flow streams as shown in Exhibit 9.
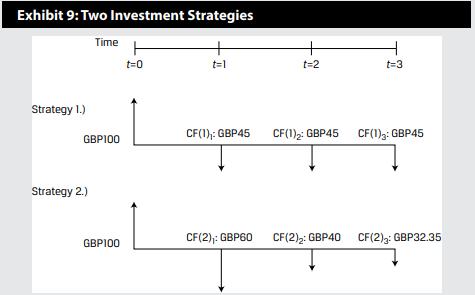
Your required return for both investment strategies is 10 percent per time period.
1. Recommend which investment strategy to choose.
Transcribed Image Text:
Exhibit 9: Two Investment Strategies Strategy 1.) Time GBP100 Strategy 2.) GBP100 t=0 t=1 CF(1),: GBP45 + t=2 t=3 CF(1)2: GBP45 CF(1): GBP45 CF(2),: GBP60 CF(2)2: GBP40 CF(2): GBP32.35
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
1 To make a recommendation between these two strategies we need to establish which one has the highe...View the full answer

Answered By

Carly Cimino
As a tutor, my focus is to help communicate and break down difficult concepts in a way that allows students greater accessibility and comprehension to their course material. I love helping others develop a sense of personal confidence and curiosity, and I'm looking forward to the chance to interact and work with you professionally and better your academic grades.
4.30+
12+ Reviews
21+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Business questions
-
Let r and s be solutions to the quadratic equation x 2 b x + c = 0. For n N, define d0 = 0 d1 = r s dn = b dn1 c dn2 (n 2) Prove that dn = r n s n for all n N. [4 marks] (b) Recall that a commutative...
-
In this question assume that p and q are atomic formulae. (a) Compare and contrast path formulae and state formulae in temporal logic. [4 marks] (b) Describe and contrast the meanings of F(G p) and...
-
Predictive text entry systems are familiar on touch screens and mobile phones. This question asks you to consider how the same principles might be used in a programming editor for creating Java code....
-
Although epoxides are always considered to have their oxygen atom as part of a threemembered ring, the prefix epoxy in the IUPAC system of nomenclature can be used to denote a cyclic ether of various...
-
For a 12% interest rate, compute the value of F in the following diagram. 200 100 200
-
Have you ever gone to a hotel spa? Why do you think an increasing number of hotels have added a spa to their facility?
-
Describe international distribution. LO.1
-
The cycle division of TravelFast Company has the following cost data per unit for its most recent cycle, the Roadbuster: The cycle division currently buys its body frames from an outside supplier....
-
Hart, Attorney at Law, experienced the following transactions in Year 1, the first year of operations: 1. Accepted $18,400 on April 1, Year 1, as a retainer for services to be performed evenly over...
-
A stock currently trades at USD25. In one year, it will either increase in value to USD35 or decrease to USD15. An investor sells a call option on the stock, granting the buyer the right, but not the...
-
1. Suppose Coca-Cola stock trades at a forward price to earnings ratio of 28, its expected dividend payout ratio is 70 percent, and analysts believe that its dividend will grow at a constant rate of...
-
Preparing journal entries for income tax expense. The income tax note to the financial statements of L.A. Gear for three recent years reveals the following (amounts in thousands): The deferred taxes...
-
How do you demonstrate resilience as a leader during times of crisis or uncertainty, and what steps do you take to bolster your team's resilience ?
-
What would you do if it becomes clear to you that the potential successor you were grooming is not going to make the grade as a supervisor? What are your next steps? Do you think this grooming is...
-
How do services and products differ? What kind of decisions do companies make regarding products and services? Why are brands important to marketers? How do marketing strategies change during the...
-
What leadership principles do you feel you possess that are important for APRNs to exhibit? What principles do you need to explore to be more confident in performing? Which leadership style do you...
-
Question 1- Where do you go in the Courier to find out your amount of leftover inventory for a specific product last round? Based on the production tab of the worksheet I gave you; how do you use...
-
Estimate the enthalpy of reaction R for the combustion process of carbon monoxide at 1800 K, using (a) Enthalpy data (b) KP data.
-
Identify the Critical Infrastructure Physical Protection System Plan.
-
If sales promotion spending continues to grow often at the expense of media advertis inghow do you think this might affect the rates charged by mass media for advertising time or space? How do you...
-
As a community service, disc jockeys from radio station WMKT formed a basketball team to help raise money for local nonprofit organizations. The host organization finds or fields a competing team and...
-
How should the acceptance of a profit-oriented, a sales-oriented, or a status quooriented pricing objective affect the development of a companys marketing strategy? Illustrate for each.
-
Your company produces a health magazine. Its sales data for 1 - year subscriptions are as follows: Year of Operation Subscriptions Sold % Expired at Year End 2 0 2 0 $ 3 0 0 , 0 0 0 5 0 2 0 2 1 $ 6 4...
-
The adjusted trial balance for Tybalt Construction on December 3 1 of the current year follows. TYBALT CONSTRUCTION Adjusted Trial Balance December 3 1 Number Account Title Debit Credit 1 0 1 Cash $...
-
( US$ millions ) 1 2 / 3 1 / 2 0 1 4 1 2 / 3 1 / 2 0 1 3 1 2 / 3 1 / 2 0 1 2 1 2 / 3 1 / 2 0 1 1 Net income $ 1 4 , 4 3 1 $ 1 2 , 8 5 5 $ 1 0 , 7 7 3 $ 9 , 7 7 2 Depreciation 3 , 5 4 4 2 , 7 0 9 1 ,...

Study smarter with the SolutionInn App


