Question: a. Use Troutons rule, Hvap (88 J mol-1 K-1) H vap (88 J mol -1 K -1 ) to estimate the enthalpy of
a. Use Trouton’s rule, ΔHvap° ≈ (88 J mol-1 K-1) ΔH°vap ≈ (88 J mol-1 K-1) to estimate the enthalpy of vaporization of octane (b.p. 126°C). (b.p. 126°C)
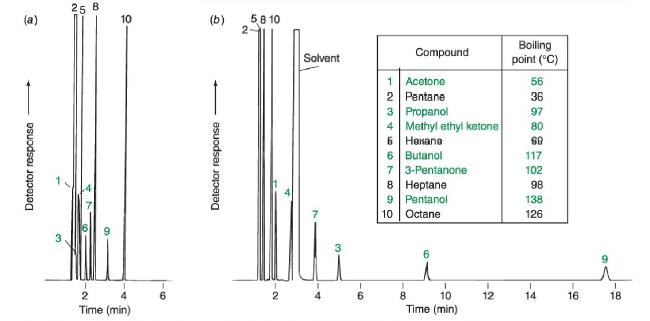
b. Use the form of the Clausius-Clapeyron equation below to estimate the vapor pressure in bar of octane at the column temperature in Figure 24-9 (70°C).(70°C).
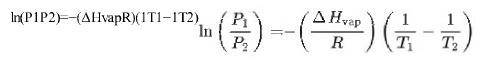 c. Calculate the vapor pressure in bar for hexane (b.p. 69°C)(b.p. 69°C) at 70°C.70°C.
c. Calculate the vapor pressure in bar for hexane (b.p. 69°C)(b.p. 69°C) at 70°C.70°C.
d. What is the relationship between solute vapor pressure and retention?
e. Why is the technique called “gas chromatography” if retained analytes are only partially vaporized?
Figure 24-9
A IL (17) -- (^) ( 1 - 12) In R T T In(P1P2)-(AHvapR)(1T1-1T2)
Step by Step Solution
3.44 Rating (151 Votes )
There are 3 Steps involved in it
Answer a Using Troutons Rule the enthalpy of vaporization of octane is estimated ... View full answer

Get step-by-step solutions from verified subject matter experts


