Question: Blank solutions were monitored for vanadium as 51V+ 51 V + using inductively coupled plasma-mass spectrometry with the following results. a. Is the HClHCl used
Blank solutions were monitored for vanadium as 51V+ 51V+ using inductively coupled plasma-mass spectrometry with the following results.
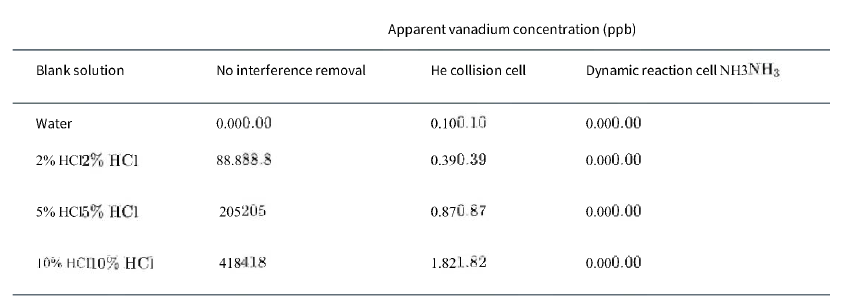
a. Is the HClHCl used to prepare the blank solutions contaminated with vanadium?
b. Would monitoring 50V+50V+ be a better alternative?
c. What acid should be used in place of HClHCl?
Blank solution Water 2% HC12% HC1 5% HCI5% HC1 10% HC110% HCI No interference removal 0.000.00 88.888.8 205205 418418 Apparent vanadium concentration (ppb) He collision cell 0.100.10 0.390.39 0.870.87 1.821.82 Dynamic reaction cell NH3NH3 0.000.00 0.000.00 0.000.00 0.000.00
Step by Step Solution
3.49 Rating (175 Votes )
There are 3 Steps involved in it
Answer a No the HClHCl used to prepare the blank s... View full answer

Get step-by-step solutions from verified subject matter experts


