Consider the separation of inorganic and organic anions in Figure 26-7. a. What is the probable charge
Question:
Consider the separation of inorganic and organic anions in Figure 26-7.
a. What is the probable charge (Xn-) (Xn-)of pyruvate (peak 1010), 2-2-oxovalerate (peak 1616), and maleate (peak 2828)?
b. Iodide (peak 44 44) is a -1-1 ion. Explain its strong retention.
Figure 26-7
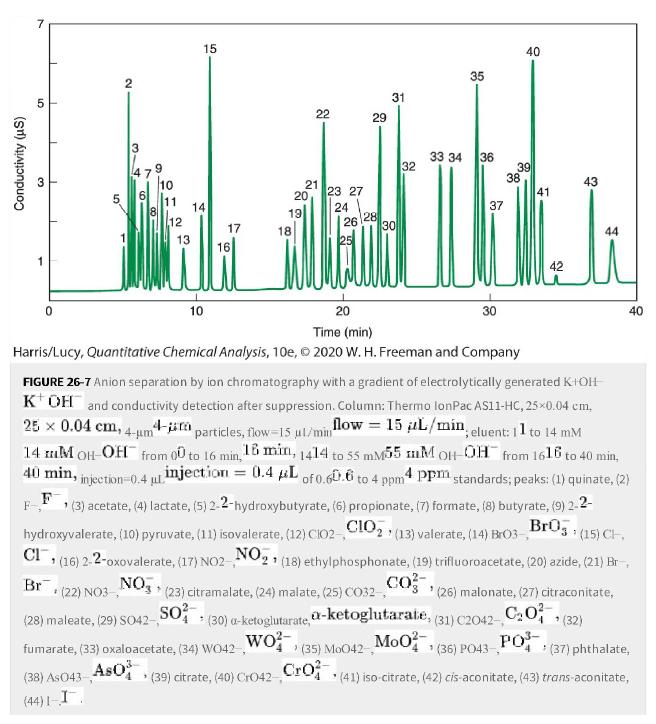
Transcribed Image Text:
Conductivity (us) 31 3 T 50 2 3 A 6 9 10 11 15 12 17 ili 10 19 18 20 22 21 23 27 24 26 29 28 31 . (39) citrate, (40) Cro42 Cro. 30 32 (35) Mo042- 33 34 35 36 37 1 30 20 Time (min) Harris/Lucy, Quantitative Chemical Analysis, 10e, © 2020 W. H. Freeman and Company 39 38 40 41 43 44 FIGURE 26-7 Anion separation by ion chromatography with a gradient of electrolytically generated K+OH KOH and conductivity detection after suppression. Column: Thermo lonPac AS11-HC, 25×0.04 cm, 25 x 0.04 cm, 4-um4 4- particles, flow-15 µl/min flow = 15 L/min, eluent: 11 to 14 mM 14 M OH-OH from 0 to 16 min, 18 min, 1414 to 55 mM55 mM OH-OH from 1616 to 40 min, 40 min, injection 0.4 injection = 0.4 μL of 0.60.6 to 4 ppm4 ppm standards; peaks: (1) quinate, (2) (3) acetate, (4) lactate, (5) 2-2-hydroxybutyrate, (6) propionate, (7) formate, (8) butyrate, (9) 2-2- F hydroxyvalerate, (10) pyruvate, (11) isovalerate, (12) C102 CIO₂, (13) valerate, (14) Bro3_BrO (15) CH CI, (16) 2-2-oxovalerate, (17) NO2 NO₂ (18) ethylphosphonate, (19) trifluoroacetate, (20) azide, (21) Br NO (23) citramalate, (24) malate, (25) CO32- CO (30) a-ketoglutarate, a-ketoglutarate, wo²- Br (22) NO3-, (28) maleate, (29) SO42-,- fumarate, (33) oxaloacetate, (34) WO42, AsO SO² Mo0² (38) As 043- (44)1-I. 40 (26) malonate, (27) citraconitate, (31) C2042-C₂0² * (32) PO³ 1 (36) PO43- * (41) iso-citrate, (42) cis-aconitate, (43) trans-aconitate, > (37) phthalate,
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
Answer a The probable charge of pyruvate peak 1010 is 2 the probable charge of ...View the full answer

Answered By

Justin Akengo
I am writing in application for the tutor position with your organisation. I am experienced in tutoring students of all abilities and I believe I am the ideal candidate for this position.
I work with students of all ages, from elementary school to college level. Whether the subject is science, Mathematics or basic study skills, I break material down into easy-to-understand concepts. In your job posting, you asked for someone who can tutor in a variety of subjects. I am comfortable explaining calculus to a college student or working with a kindergartener on spelling fundamentals.
Below are just a few core skills and qualifications I posses as a tutor;
Adept at creating study materials in a variety of academic subjects to help students improve their test scores and GPAs.
Strong interpersonal skills in working with students to help them achieve and succeed.
Have written study books adopted by a high school and a college to help students improve their skills in English and mathematics.
Have won several “Tutor of the Year” awards for work with high school and college students.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Quantitative Chemical Analysis
ISBN: 9781319164300
10th Edition
Authors: Daniel C. Harris, Charles A. Lucy
Question Posted:
Students also viewed these Sciences questions
-
Iodide ion is oxidized by hypochlorite ion in basic solution. I(aq) + ClO(aq) Cl(aq) + IO(aq) In 1.00 M NaOH at 25oC, the iodide-ion concentration (equal to the ClO concentration) at different times...
-
Consider the reaction of peroxydisulfate ion (S2O8-2) with iodide ion (I) in aqueous solution: S2O82- (aq) + 3 I (aq) - 2 SO42- (aq) + I3(aq) At a particular temperature the initial rate of...
-
The iodide ion catalyzes the decomposition of aqueous hydrogen peroxide, H2O2. This decomposition is believed to occur in two steps. H2O2 + I H2O + IO (elementary reaction) H2O2 + IO H2O + O2 + I...
-
Clara Hughes, who is pushing 5 0 , has medaled both in speedskating and road cycling ( and showing no signs of slowing down ) completed a training event where she biked 5 0 km east, stopped and rode...
-
For the conditions of Exercise 7 and the data given in Table 11.1, determine the value of R2, as defined by Eq. (11.5.26).
-
In the audit of the Worldwide Wholesale Company, you did extensive ratio and trend analysis as part of preliminary audit planning. Your analytical procedures identified the following: 1. Commission...
-
(b) Analysing measurements made on each occasion separately, and using the block factor and the treatment factor as model terms, perform analyses of variance on the measurements of fibre diameter...
-
Presented below are two independent revenue arrangements for Colbert Company. Instructions Respond to the requirements related to each revenue arrangement. (a) Colbert sells 3D printer systems....
-
Future Amount i = n = Present Value $ 89,000 11% 79,000 11% 69,000 11% 59,000 11% 49,000 11% 790,000 11% $ 0 Should the restaurant be purchased? Question Helga is considering the purchase of a small...
-
Jamal took over as the general manager of High view Clique Resort Ltd., a famous tourist hotel in October 2020. At the time, the salaries to employees were six months in arrears. The hotel rooms,...
-
Isocratic separation with HPLC Teaching Assistant, an Excel spreadsheet that simulates a reversed-phase liquid chromatographic separation. Download the Excel file from SaplingPlus,...
-
Simulating a separation with a spreadsheet. Use the spreadsheet in Figure 25-39 to simulate the chromatograms for =0.75= 0.75 and =0.56= 0.56 in Figure 25-40. Figure 25-39 Figure 25-40 A 1...
-
Write an essay advocating the case for flotation on a recognised investment exchange.
-
Assume you have been given $400,000 CAD with access to all listed stocks, bonds, futures, and options worldwide. You can trade in options and futures, in combination with the underlying asset....
-
Charlene wrote a letter to Rachel offering to sell her car, a Proton Saga, for RM 60,000. The letter reached Rachel on 25. 11.2020. Rachel sent her letter of acceptance at 3 p.m. on the same day....
-
Data for the risk premium sensitivities (b, s, and h) as well as the beta coefficient for the CAPM of two companies are listed in the following table: Company b s h ERP SMBP HMLP Beta Alpha 1.1114...
-
Free-Response Questions 1. m Initial position eviribrA ARAL m Incline raised to 0 <0max pr A block of mass m is initially at rest on a rough board, which is initially horizontal on a tabletop. The...
-
A picture frame sits atop a bookshelf. When the bookshelf is bumped, the frame tumbles to the floor, landing after 0.64 s. How tall is the bookshelf?
-
A siren is approaching you at 35 m/s and is perceived to have a frequency of 1100 Hz. If the siren stops moving, what frequency do you now hear?
-
Explain briefly what is meant by electronic data interchange (EDI). How does EDI affect a companys audit trail?
-
Find the absolute and percent relative uncertainty and express each answer with a reasonable number of significant figures. (a) 9.23 ( 0.03) + 4.21 ( 0.02) - 3.26 ( 0.06) = ? (b) 91.3 ( 1.0) 40.3 (...
-
Verify the following calculations: (a) 3.1415( 0.0011) = 1.77243( 0.00031) (b) log[3.1415 ( 0.0011)] = 0.49714 ( 0.00015) (c) antilog[3.141.5 ( 0.0011)] = 1.3852( 0.0035) 103 (d) ln[3.1415 (...
-
Verify the following calculations: (a) 3.1415( 0.0011) = 1.77243( 0.00031) (b) log[3.1415 ( 0.0011)] = 0.49714 ( 0.00015) (c) antilog[3.141.5 ( 0.0011)] = 1.3852( 0.0035) 103 (d) ln[3.1415 (...
-
The market price of a stock is $24.55 and it is expected to pay a dividend of $1.44 next year. The required rate of return is 11.23%. What is the expected growth rate of the dividend? Submit Answer...
-
Suppose Universal Forests current stock price is $59.00 and it is likely to pay a $0.57 dividend next year. Since analysts estimate Universal Forest will have a 13.8 percent growth rate, what is its...
-
ABC Company engaged in the following transaction in October 2 0 1 7 Oct 7 Sold Merchandise on credit to L Barrett $ 6 0 0 0 8 Purchased merchandise on credit from Bennett Company $ 1 2 , 0 0 0 . 9...

Study smarter with the SolutionInn App


