Question: Examine the procedure in Table 7-1 for the Fajans titration of Zn2+.Zn 2+ . Do you expect the charge on the precipitate to be positive
Examine the procedure in Table 7-1 for the Fajans titration of Zn2+.Zn2+. Do you expect the charge on the precipitate to be positive or negative after the equivalence point?
Table 7-1
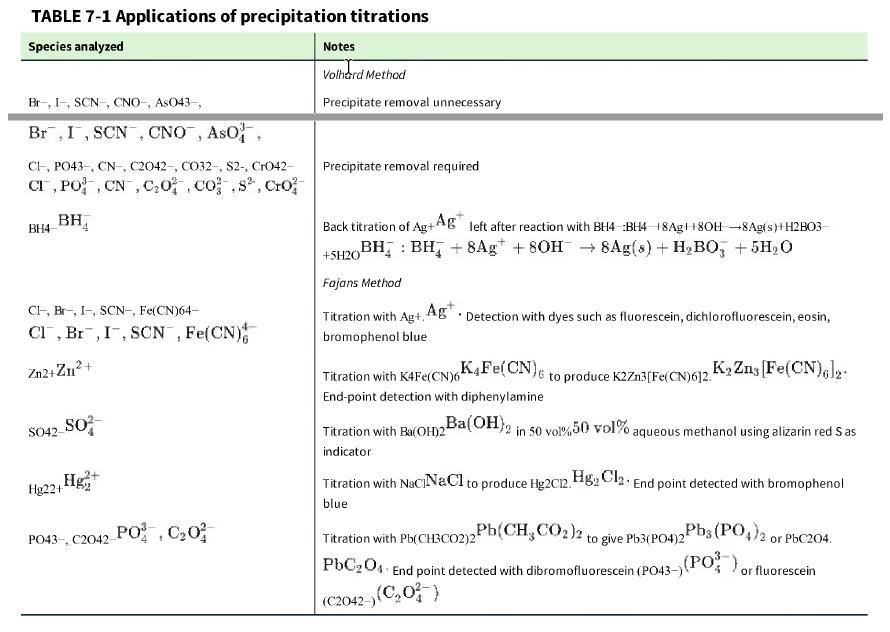
TABLE 7-1 Applications of precipitation titrations Species analyzed Br-, I-, SCN-, CNO-, AsO43-, Br, I, SCN, CNO, ASO, CI, PO43-, CN, C2042-, CO32-, S2-, CrO42- CI, PO, CN, C0, cos, Cro BH4 BH Cl, Br, I-, SCN-, Fe(CN)64- Cl, Br, I, SCN, Fe(CN) Zn2+Zn+ SO42- SO Hg+ Hg22+1 PO43-, C2042-PO, C0 Notes Volhard Method Precipitate removal unnecessary Precipitate removal required Back titration of Ag+Ag left after reaction with BH4 BH4 18Ag8OH-8Ag(s)+H2B03- BH BH + 8Ag +80H 8Ag(s) + HBO3 + 5HO +5H201 Fajans Method Titration with Ag+, Ag. Detection with dyes such as fluorescein, dichlorofluorescein, eosin, bromophenol blue to produce K2Zn3[Fe(CN)612. K Zns [Fe(CN)6]2. Ba(OH)2 in 50 vol%50 vol% aqueous methanol using alizarin red Sas K4Fe(CN)6 Titration with K4Fe(CN)6 End-point detection with diphenylamine Titration with Ba(OH)2 indicator Titration with NaciNaCl to produce Hg2012. HgCl End point detected with bromophenol blue Pb(CH3CO2)2 to give Pb3(PO4)2 Titration with Pb(CH3CO2)2 PbC204 End point detected with dibromofluorescein (PO43-) (C2042-) (C0) Pb3(PO4)2 or PbC204. (PO), or fluorescein
Step by Step Solution
3.34 Rating (154 Votes )
There are 3 Steps involved in it
The Fajans titration of Zn2 produces a precipitate ... View full answer

Get step-by-step solutions from verified subject matter experts


