Question: Formaldehyde has a nominal (integer) mass of 30 Da, 30 Da, but its mass spectrum in Figure 22-7 has seven peaks at different values of
Formaldehyde has a nominal (integer) mass of 30 Da, 30 Da, but its mass spectrum in Figure 22-7 has seven peaks at different values of m/z m/z. Explain the origin of each peak. Draw a Lewis dot structure of each ion to decide which ones have an unpaired electron.
Figure 22-7
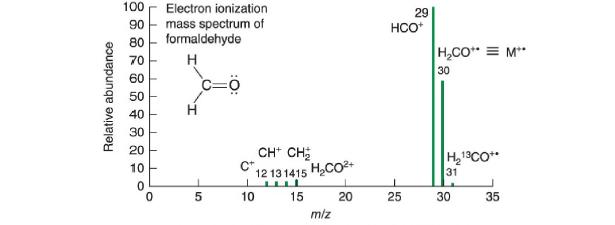
Relative abundance 100 Electron ionization mass spectrum of formaldehyde H 90 80 70 60 50 40 30 20 10 0 0 H 5 =0 CH CH 12131415 HCO 15 C+ I 10 m/z 20 29 HCO+ 25 HCO = M** 30 H13CO** 31 30 35
Step by Step Solution
3.40 Rating (162 Votes )
There are 3 Steps involved in it
Answer The seven peaks in the mass spectrum of formaldehyde correspond to different ionic ... View full answer

Get step-by-step solutions from verified subject matter experts


