Question: In the spectrum below, identify as many peaks as you can in the X-ray fluorescence spectrum of soil from the location where water from the
In the spectrum below, identify as many peaks as you can in the X-ray fluorescence spectrum of soil from the location where water from the roof of a house drips onto the ground. For each KαKα peak, state where the KβKβpeak should appear, and state whether there is a plausible peak at the KβKβ position. There must be a KβKβ for every KαKα, but the KβKβ intensity should be only ~1/5 - 1/5 of the KαKα intensity.
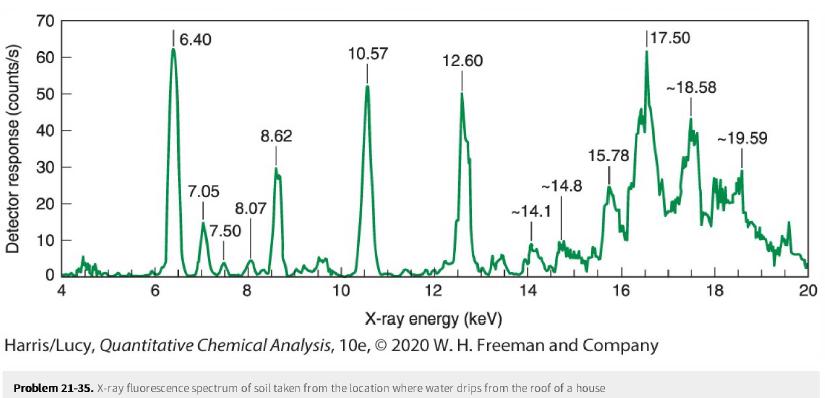
Detector response (counts/s) 70 60 50 40 30 20 10 0 T T T 1 1 4 6 6.40 7.05 | 8.07 8.62 7.50 8 10.57 10 12.60 12 -14.8 -14.1 wit 14 15.78 16 Problem 21-35. X-ray fluorescence spectrum of soil taken from the location where water drips from the roof of a house 17.50 X-ray energy (keV) Harris/Lucy, Quantitative Chemical Analysis, 10e, 2020 W. H. Freeman and Company -18.58 -19.59 18 20
Step by Step Solution
3.45 Rating (161 Votes )
There are 3 Steps involved in it
In this Xray fluorescence spectrum there are several peaks that can be identified There is a peak at around 34 keV which is likely the K peak for the ... View full answer

Get step-by-step solutions from verified subject matter experts


