Question: Use Figure 25-33 for the following questions: a. What pHpH would be best for the separation of benzoic acid, 4-4-nitrophenol, and 3-3-methylbenzoic acid? b. What
Use Figure 25-33 for the following questions:
a. What pHpH would be best for the separation of benzoic acid, 4-4-nitrophenol, and 3-3-methylbenzoic acid?
b. What pHpH would be best for the separation of benzoic acid, 3-3-methylbenzoic acid, and 4-4-methylaniline?
c. What pHpH would be best for separation of 4-4-nitrophenol, 4-4-methylaniline, and codeine on a typical C18-silica C18-silica column?
Figure 25-33
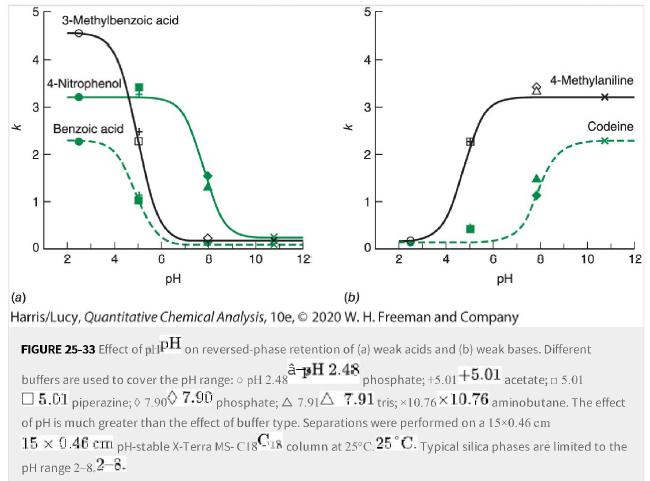
K 3 2 1 3-Methylbenzoic acid 4-Nitrophenol Benzoic acid 2 G 0 10 12 pH K 5 4 3 2 1 0 I 2 4-Methylaniline Codeine 10 12 pH (b) (a) Harris/Lucy, Quantitative Chemical Analysis, 10e, 2020 W. H. Freeman and Company FIGURE 25-33 Effect of plPH on reversed-phase retention of (a) weak acids and (b) weak bases. Different pH 2.48 buffers are used to cover the pH range: o pH 2.48 phosphate; +5.01+5.01 acetate; n 5.01 5.01 piperazine; 7.900 7.90 phosphate; A 7.91A 7.91 tris; 10.7610.76 aminobutane. The effect of pH is much greater than the effect of buffer type. Separations were performed on a 15x0.46 cm 15 x 0.46 cm pH-stable X-Terra MS-C1818 column at 25C 25 C. Typical silica phases are limited to the pH range 2-8.2-8.
Step by Step Solution
3.46 Rating (162 Votes )
There are 3 Steps involved in it
Answer a The best pH for the separation of benzoic acid 44nitroph... View full answer

Get step-by-step solutions from verified subject matter experts


