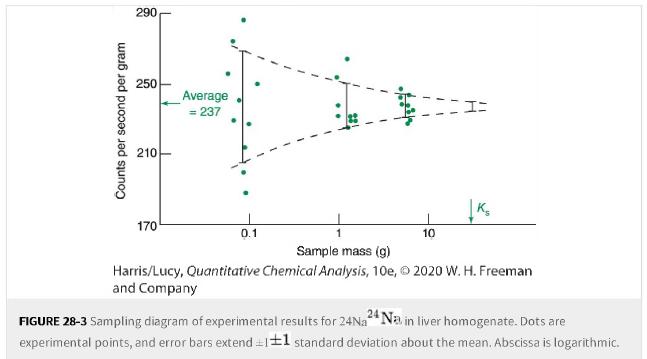
What mass of sample in Figure 28-3 is expected to give a sampling standard deviation of 6%?6%?
Question:
What mass of sample in Figure 28-3 is expected to give a sampling standard deviation of ±6%?±6%?
Figure 28-3
Transcribed Image Text:
Counts per second per gram 290 250 210 170 Average = 237 0.1 10 K₂ Sample mass (g) Harris/Lucy, Quantitative Chemical Analysis, 10e, 2020 W. H. Freeman and Company FIGURE 28-3 Sampling diagram of experimental results for 24Na 24 Na in liver homogenate. Dots are experimental points, and error bars extend = 1±1 standard deviation about the mean. Abscissa is logarithmic.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 62% (16 reviews)
Calculations The mass of sample in Figure 283 is expected to give ...View the full answer

Answered By

Labindao Antoque
I graduated in 2018 with a Bachelor of Science degree in Psychology from Dalubhasaan ng Lungsod ng San Pablo. I tutored students in classes and out of classes. I use a variety of strategies to tutor students that include: lecture, discussions about the subject matter, problem solving examples using the principles of the subject matter being discussed in class , homework assignments that are directed towards reinforcing what we learn in class , and detailed practice problems help students to master a concept. I also do thorough research on Internet resources or textbooks so that I know what students need to learn in order to master what is being taught in class .
0.00
0 Reviews
10+ Question Solved
Related Book For 

Quantitative Chemical Analysis
ISBN: 9781319164300
10th Edition
Authors: Daniel C. Harris, Charles A. Lucy
Question Posted:
Students also viewed these Sciences questions
-
Louvre Inc. bought a business that is expected to give a 25% annual rate of return on the investment. Of the total amount paid for the business, $75,000 was deemed to be goodwill, and the rest was...
-
Which of the following would be expected to give a positive test with Benedict's reagent? Why? (a) L-Arabinose (b) 1,3-Dihydroxyacetone (c) D-Fructose (d) Lactose (e) Amylose
-
What are the mean and standard deviation of the sampling distribution of x ? What are the mean and standard deviation of the sampling distribution of p?
-
1. A projectile is launched in a vertical plane, at an angle 0 with initial velocity vo. It must be caught in a frictionless circular tube of radius R in such a way that the trajectory of the...
-
Suppose that X1, . . . , Xm form a random sample from a continuous Distribution for which the p.d.f. f (x) is unknown; Y1, . . . , Yn form an independent random sample from another continuous...
-
Woodinville Cement uses a process costing system. In 2020, the company produced and sold 100,000 bags of cement and incurred the following costs: The current selling price is $4 per unit, and the...
-
(c) Arrange the data for analysis by GenStat, R or SAS.
-
Bonds of Zello Corporation with a par value of $1,000 sell for $960, mature in 5 years, and have a 7% annual coupon rate paid semiannually. a. Calculate each of the following yields: i. Current...
-
The higher the discount rate, the greater the importance of the early cash flows. (Select the best choice below.) A. False OB. True
-
A Pelton wheel is to be designed to develop 735.5 kW at 400 r.p.m. It is to be supplied with water from a reservoir whose level is 250 m above the wheel through a pipe 900 m long. The pipeline losses...
-
Cloud-point extraction. Traces of lead in drinking water cause adverse health effects. The micelle-forming surfactant Triton X-114 (plus the co-surfactant cetyl trimethylammonium bromide) and a crown...
-
In the chapter opener, trace metals in teeth and bone provide information about Otzi the Icemans diet and environment as a child and adult. The inorganic mineral matrix of teeth and bone is...
-
Describe when to include the value of non-operating assets in a valuation of minority equity interests.
-
10.1 Learning Outcomes: Describe managers' appropriate use of power and influence. Identify traits and characteristics of successful leaders. Identify behaviors of successful leaders 10.2 Action...
-
Suppose Ron went to Rio de Janeiro in 2019 when the dollar was worth 4 reals. The price of a cup of coffee at Starbucks in the US was $4, so when Ron converted $4 into reals he had 16 reals, which...
-
The expected annual net income is $200,000; the average investment is $800,000; and depreciation expense is $50,000. Calculate the annual rate of return.
-
How do you define humanities?
-
Write the types of partners ?
-
A source of sound has a total emitted power of 300 W. What is the intensity a distance of 10 m from the source? Assume the source emits spherical waves.
-
Flicker, Inc., a closely held corporation, acquired a passive activity this year. Gross income from operations of the activity was $160,000. Operating expenses, not including depreciation, were...
-
Calculate how many milliliters of 0.626 M KOH should be added to 5.00 g of MOBS (Table 8-2) to give a pH of 7.40.
-
(a) Use Equations 8-20 and 8-21 to find the pH and concentrations of HA and A- in a solution prepared by mixing 0.00200 mol of acetic acid plus 0.004 00 mol of sodium acetate in 1.00 L of water. (b)...
-
(a) Calculate the pH of a solution prepared by mixing 0.0100 mol of the base B (Kb = 10 = - 2.00) with 0.020 0 mol of BH+Br- and diluting to 1.00 L. First calculate the pH by assuming [B] = 0.0100...
-
A government bond matures in 30 years, makes semi-annual coupon payments of 6.0% ($120 per year) and offers a yield of 3.7% annually compounded. Assume face value is $1,000. Three years later the...
-
Your objective is: 1. Carry out a life insurance needs analysis, for each one of them (show your calculations) [30 Marks] 2. Refer to the case and the insurance plan quotes. Would you recommend...
-
TufStuff, Incorporated, sells a wide range of drums, bins, boxes, and other containers that are used in the chemical industry. One of the company s products is a heavy - duty corrosion - resistant...

Study smarter with the SolutionInn App


