1. Identify the electrophilic site in each of the following molecules by selecting e electrophilic atom. 2. 2. For the Bronsted acid-base reaction shown below,
1. Identify the electrophilic site in each of the following molecules by selecting e electrophilic atom.
 2.
2.
2. For the Bronsted acid-base reaction shown below, determine the conjugate acid-base pairs.
Then give the curved-arrow notation for the reaction in the left-to-right direction. (To draw the arrows, click on the reaction to get into the edit mode, then click on the curved arrow icon.)
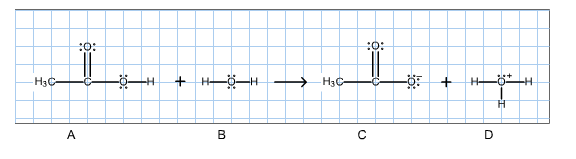
Fill in the blanks with the letters A,B,C and D, representing the species in the reaction above.

For the following reaction, indicate which reactant is the Lewis acid an which is the Lewis base.
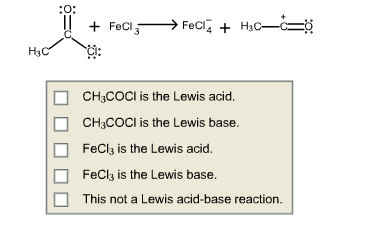
4.
Determine the dissociation constants for the following acids. Express the answers in proper scientific notation where appropriate.
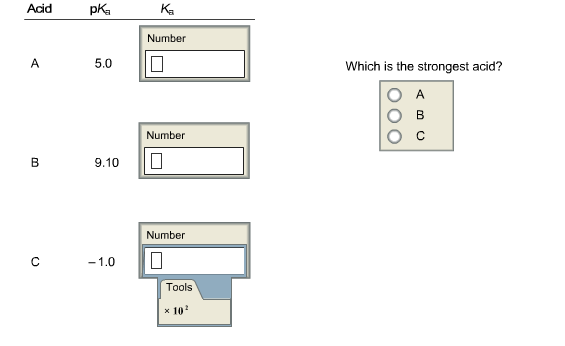
5. Complete the equations for the following equilibria and calculate Kea where the Keq expression includes IF1201. Be sure to enter Keg in proper scientific notation.
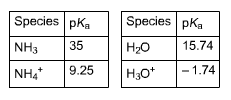
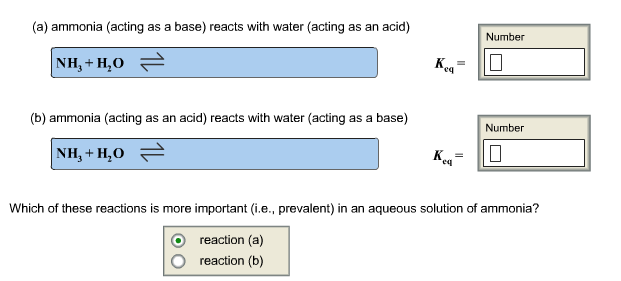
Click on an atom to select it; click again to deselect it. A selected atom will be colored green. H3C- A :0: -O-H + H-O-H B -H3C- :0: C 16: + HH H cid and its conjugate base acid and its conjugate base H3C :0: || + FeCl3 FeCl + HC-C8 CH3COCI is the Lewis acid. CH3COCI is the Lewis base. FeCl3 is the Lewis acid. FeCl3 is the Lewis base. This not a Lewis acid-base reaction. Acid A pK 5.0 B 9.10 -1.0 Ka Number Number Number Tools 102 Which is the strongest acid? A B Species pKa NH3 35 NH4* 9.25 Species pKa HO H3O+ 15.74 -1.74 (a) ammonia (acting as a base) reacts with water (acting as an acid) NH,+H,O = (b) ammonia (acting as an acid) reacts with water (acting as a base) | NH,+H,O Keq Kea 10. Number 10 Number Which of these reactions is more important (i.e., prevalent) in an aqueous solution of ammonia? reaction (a) reaction (b)
Step by Step Solution
3.53 Rating (150 Votes )
There are 3 Steps involved in it
Step: 1
Part 1 Draw the given molecule Explanation The given compounds are drawn in figure 1 Indicate the electrophilic site in both compounds The electrophilic atoms are colored green Explanation The carbon ...
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


