Question
Because of environmental considerations, acetone must be removed from air used in your chemical plant before the air is released into the atmosphere. The acetone
Because of environmental considerations, acetone must be removed from air used in your chemical plant before the air is released into the atmosphere. The acetone is removed by absorbing it into water and distilling the water to produce and acetone-rich stream and a water-rich stream.
Two streams enter the process as listed in the table below. The first is the dirty air stream, which has a total mass flow rate of 3500 kg/hr. The second it a pure water stream.
Three streams exit the process as shown in the table, a pure air strearn, an acetone-rich liquid. and a water rich-liquid. The flow rate of the water-rich outlet stream has been measured to be 650 kg/hr.
a) What arc the mass flow rates of the other streams?
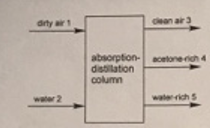
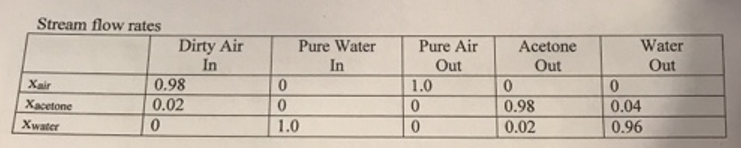
dirty air 1 water 2 clean air 3 absorption- acetone-rich 4 distillation column water-rich 5 Stream flow rates Xair Xacetone Xwater Dirty Air In 0.98 0.02 0 Pure Water In 0 0 1.0 Pure Air Out 1.0 0 0 Acetone Out 0 0.98 0.02 Water Out 0 0.04 0.96
Step by Step Solution
3.37 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1
Assuming that the given composition is in mass fra...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


