Question
Calculate ? H rxn for the combustion of octane (C 8 H 18 ), a component of gasoline, by using average bond energies. Calculate ?
Calculate ? H rxn for the combustion of octane (C 8 H 18 ), a component of gasoline, by using average bond energies.
Calculate ? H rxn for the combustion of octane by using enthalpies of formation from Appendix IIB in the textbook. The standard enthalpy of formation of C 8 H 18 is -250 kJ/mol.
What is the percent difference between the two results?
Which result would you expect to be more accurate?
The value calculated from the heats of formation
The value calculated from the average bond energies
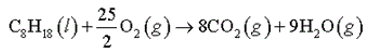
(8)0H6+(3)008 (8) 0+ (1) H)
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Combustion of liquid octane is as follows The standard enthalpy of reaction c...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Thermodynamics for Engineers
Authors: Kenneth A. Kroos, Merle C. Potter
1st edition
1133112862, 978-113311286
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



