Question
Determine the value of G for the reaction of the fuel gas acetylene with hydrogen gas: C 2 H 2 (g) + 2H 2 (g)
Determine the value of ΔG° for the reaction of the fuel gas acetylene with hydrogen gas:
C2H2 (g) + 2H2 (g) —> C2H6 (g)given the following thermodynamic properties at 25°C:
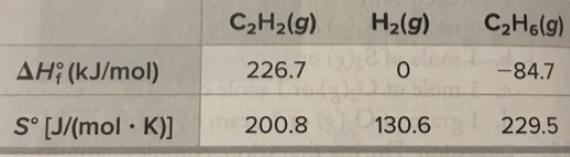
C2H2(g) H2(g) C2H6(g) AH; (kJ/mol) 226.7 -84.7 S [J/(mol K)] 200.8 130.6 229.5
Step by Step Solution
3.47 Rating (160 Votes )
There are 3 Steps involved in it
Step: 1
C2H2 2H2 C2H6 delta H for reaction delta H C2H6 delta H C2H2 2 x ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Engineering economy
Authors: Leland Blank, Anthony Tarquin
7th Edition
9781259027406, 0073376302, 1259027406, 978-0073376301
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



