Question
The following thermochemical equation is for the reaction of CO(g) with NO(g) to form CO(g) and N (9). 2CO(g) + 2NO(g) 2CO2(g) + N(g)
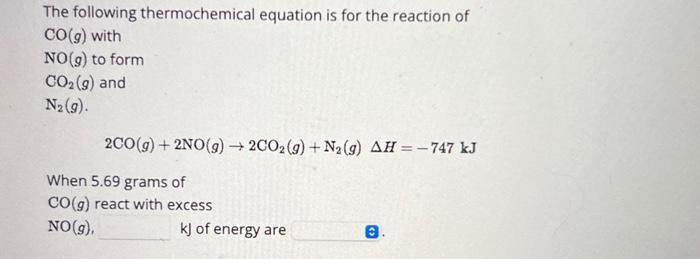
The following thermochemical equation is for the reaction of CO(g) with NO(g) to form CO(g) and N (9). 2CO(g) + 2NO(g) 2CO2(g) + N(g) AH = -747 kJ When 5.69 grams of CO(g) react with excess NO(g), kJ of energy are 0.
Step by Step Solution
3.44 Rating (160 Votes )
There are 3 Steps involved in it
Step: 1
Solutions To determine when 5699 1 first its Motor Calculate Moles of the mass amount ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
General Chemistry
Authors: Darrell Ebbing, Steven D. Gammon
9th edition
978-0618857487, 618857486, 143904399X , 978-1439043998
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



