Question
Identify the outer electron configurations for the (a) alkali metals, (b) alkaline earth metals, (c) halogens, (d) noble gases. For each electronic configuration given, choose
Identify the outer electron configurations for the (a) alkali metals, (b) alkaline earth metals, (c) halogens, (d) noble gases.
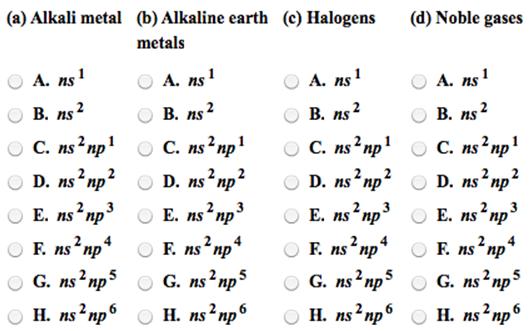
For each electronic configuration given, choose the electronic configuration of the element that would match its chemical properties.
(a)

(b)

(c)

Group the following electron configurations in pairs that would represent elements with similar chemical properties. only check there boxes.
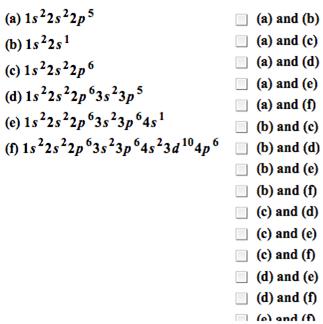
Without referring to a periodic table, write the electron configuration of the elements with the following atomic numbers.
(a) Z = 9:
(b) Z 20:
(c) Z - 26:
(d) Z - 33:
A M 2+ ion has four electrons in the 3d subshell and is derived from a metal in the first transition metal series. What element might M be?
Elemental symbol: _____
Write ground—state electron configurations for these ions, which play important roles in biochemical processes in our bodies. (Use a noble gas core abbreviation.)
(a) Na + ___
(b) mg 2+ ___
(c) CI - ___
(d) K + ___
Within each group of four atoms or ions presented below, select the species that are isoelectronic with each other:
Zn ………………………….Ge 2+
Fe 3+ ……………………….CI -
Mn 2+ ………………………B -
Ar -----------------------C
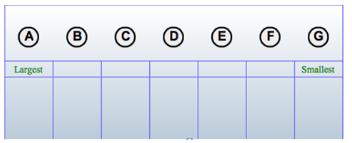
Drag each item to the correct box.
Arrange the following atoms in order of decreasing atomic radius:
A) Cs
B) K
C) Mg
D) Al
E) Na
F) CI
G) P
Classify the following bonds as covalent, polar covalent, or ionic.
(a) The bond in CsCI
Covalent
Polar
Covalent ionic
(b) The bonds in H 2 S
Covalent polar covalent ionic
(c) The NN bond in II H2 NNH2
Covalent
Polar
Covalent ionic
Draw the Lewis structure of sulfur tetrafluoride (SF4).
(a) Alkali metal (b) Alkaline earth (c) Halogens (d) Noble gases metals A. ns' A. ns! A. ns' A. ns! . ns 2 . ns 2 . ns 2 B. ns C. ns np' C. ns np' C. ns np' . ns? 2 2 D. ns np D. ns np" D. ns np? D. ns np E. ns np E. ns E. ns E. ns F. ns np* F. ns np" F. ns np O F. ns np4 G. ns np G. ns np3 G. ns? np5 O G. ns np5 6. H. ns np H. ns np H. ns np . ns * 2.
Step by Step Solution
3.54 Rating (158 Votes )
There are 3 Steps involved in it
Step: 1
1 a A b B c G d H 2 For similar chemical properties the elements should belong ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
6099b938a3918_29964.pdf
180 KBs PDF File
6099b938a3918_29964.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


