Question
Place each charge form of alanine under the pH condition where it would be the predominant form. The pKa values for the carboxyl group and
Place each charge form of alanine under the pH condition where it would be the predominant form. The pKa values for the carboxyl group and amino group of alanine are approximately 2.3 and 9.7, respectively.
If you answer any part of this question incorrectly. a single red X will appear indicating that one or more of the items were placed incorrectly. 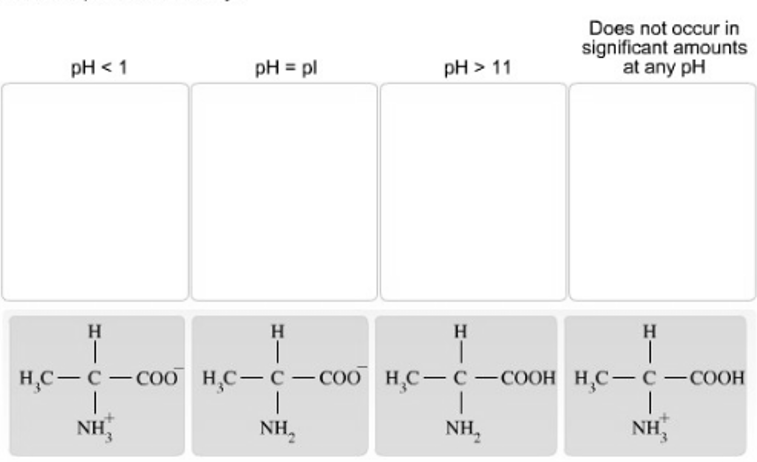 Construct a two-step synthesis of (?) leucine by dragging the appropriate formulas into the bins. Note that each bin will hold only one item. and not all of the given reagents or structures will be used.
Construct a two-step synthesis of (?) leucine by dragging the appropriate formulas into the bins. Note that each bin will hold only one item. and not all of the given reagents or structures will be used.
If one or more statements are incorrectly placed, a single red X will appear on the top left of the left bin. This does not necessarily mean the reagent placed in the left bin is wrong, but that there is a mistake somewhere. 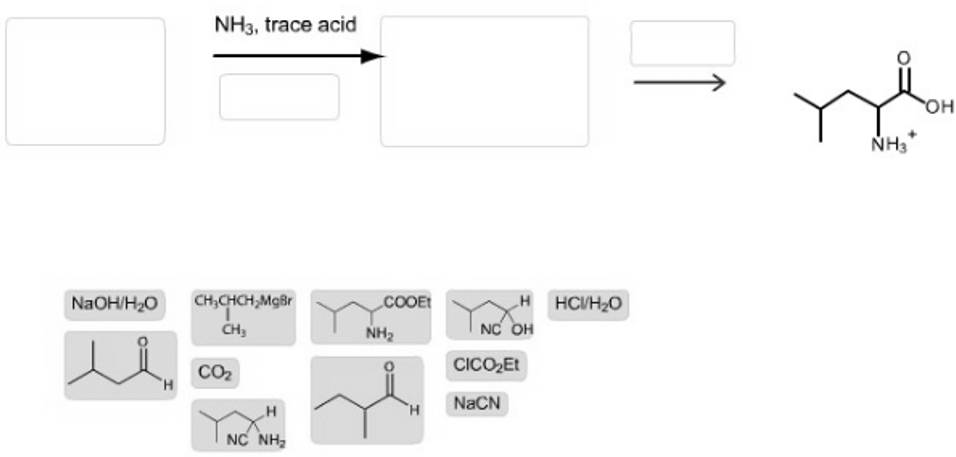
pH < 1 pH = pl H | HC-CCOO HC-C-COO NH NH, H | pH > 11 Does not occur in significant amounts at any pH H | HC-C-COOH HC-C-COOH - - NH NH H | NaOH/HO H NH3, trace acid CHCHCHMgBr CH CO H N NH, COOE NH H HCV/HO NC OH CICOEt NaCN NH3 OH
Step by Step Solution
3.47 Rating (150 Votes )
There are 3 Steps involved in it
Step: 1
In alanine pKa of carboxyl group is 23 pKa of amino group is 97 Here the pH is less than 1 Explanation The pH is less than the pKa of carboxyl group 2...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


