The data below give the dew and bubble temperature of methanoVethanol matures at 1 bar. a) A solution containing 30% methanol (by mol) is flashed
The data below give the dew and bubble temperature of methanoVethanol matures at 1 bar.
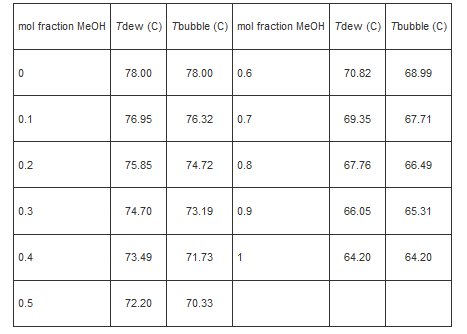
a) A solution containing 30% methanol (by mol) is flashed to 1 bar, 70.82 0 C. Determine the phase of the system; if two phases, report the compositions and relative amounts of each phase.
b) What is the maximum mol fraction of methanol that can be achieved when a solution with 30% methanol is flashed to 1 bar? The maximum mol fraction of ethanol?
c) Make a Txy plot for this system and annotate it properly.
Note: Use linear interpolations and report mol fractions to the third decimal point.
mol fraction MeOH Tdew (C) Tbubble (C) mol fraction MeOH Tdew (C) Tbubble (C) 0 0.1 0.2 0.3 0.4 0.5 78.00 76.95 75.85 74.70 73.49 72.20 78.00 0.6 76.32 74.72 71.73 0.7 73.19 0.9 70.33 0.8 1 70.82 69.35 67.76 66.05 64.20 68.99 67.71 66.49 65.31 64.20
Step by Step Solution
There are 3 Steps involved in it
Step: 1
a Bubble point is defined as the temperature at which first liquid bubble starts to evaporate Dew point is defined as the temperature at which the las...
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
609689a0d1c13_26983.pdf
180 KBs PDF File
609689a0d1c13_26983.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


