Question
The following data for water may or may not be useful for this quiz: You have a metal block with a mass of 177.1 g,
The following data for water may or may not be useful for this quiz:
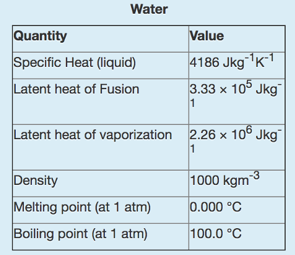
You have a metal block with a mass of 177.1 g, specific heat of 325 Jkg -1 k -1 , the thermal conductivity of 199.7 Wm -1 K -1 , an emissivity of 0.900 in air and an initial temperature of 81.7?C. The block is shaped as a square prism with a length of 5.66 cm and a cross-sectional area of 10.0 cm 2 .(that is the area of the square sides are 10.0 m 2 .) You start by dropping this metal block into an insulted 0.524 L bucket of water. The water is initially at 7.80?C.
What is the final temperature of the water?
T F = ??C
You now take the metal block and return it to its initial temperature before placing it in a room at a temperature of 14.1?C. What is the rate at this block initially loses energy? Give your answer a positive number as the block lose energy. You may assume that conservation is not important, heat is lost due to radiation.
P = ?W
What temperature would block finally reach if left in the room for a very long time?
T F = ??C
The block is now placed between a heat reservoir at 75.0?C and cold reservoir at 5.94?C (heat reservoirs are kept a constant temperature, either through them or cooling them). When the system has reached state of equilibrium what is the rate of heat flow along the block? The square sides of the block are up against the heat reservoirs.
P = ?W.
Water Quantity Specific Heat (liquid) Latent heat of Fusion Value 4186 Jkg K1 3.33 x 105 Jkg 1 Latent heat of vaporization 2.26 x 106 Jkg 1 Density Melting point (at 1 atm) Boiling point (at 1 atm) 1000 kgm-3 0.000 C 100.0 C
Step by Step Solution
3.42 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1
The expression for the heat of metal block is given as T...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


