The Grignard reaction is one of the classic methods in organic chemistry for the formation of C-C bonds. The two-step sequence involves initial reaction of
The Grignard reaction is one of the classic methods in organic chemistry for the formation of C-C bonds. The two-step sequence involves initial reaction of an organohalogen compound with magnesium in the presence of an ether solvent to form the Grignard reagent. R = X + Mg ? R ? Mg ? X
The choice of an ether solvent is important as the lone-pairs of electrons on the oxygen coordinate to the Mg and help to stabilize highly polar compound. The R-X organohalogen compound can be either an alkyl or an aryl halide, and Br is most commonly used as the halogen. The above reaction takes place at the surface of magnesium particles suspended in the solution, so proper preparation of magnesium is important to increase surface are and reduce the presence of magnesium oxide.
Due to the fact that Mg has a very low electronegativity, the carbon R chain attached to it behaves as if it has a negative charge and is therefore quite reactive as either a base or a nucleophile. Because of the strong basicity of Grignard reactions, great care must be taken to exclude water or other protic molecules as they will be readily deprotonated by the Grignard reagent. Grignard reagents may be used as nucleophiles addition reactions to C = O double bonds in compounds such as ketones, aldehydes, esters, and carbon dioxide. An alcohol is obtained once the intermediate is treated with water under acidic conditions. 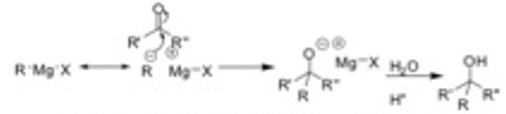
Grignard reagents arc highly reactive and can undergo undesirable sidc reactions if impurities such as water. carbon dioxide, or oxygen are found in the reaction. If the organohalogen compound is added too quickly to the magnesium. undesired coupling reactions may also occur. 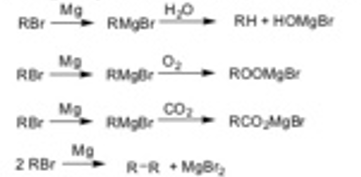
In today's experiment, you will be forming the (irignard reagent phenylmagnesium bromide by reacting bromobentene ith Mg. You will then react your Grignard reagent with benrophenone (a ketone) 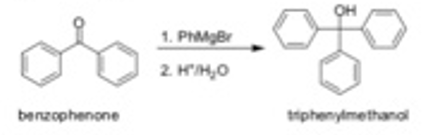 A significant factor of today's lab is that you will be responsible for planning out your reaction and determining the amounts of the starting materials to use. All students will be running the reaction using 0.015 moles of their starting carbonyl compound and a slight excess of the Grignard reagent. You will form your Grignard reagent using 1.25 molar equivalents of the bromobenzcne (relative to the benzophenone) and 1.35 molar equivalents of magnesium. You will also need to determine the best way to measure out the reagents depending on whether they are solids of liquids at room temperature.
A significant factor of today's lab is that you will be responsible for planning out your reaction and determining the amounts of the starting materials to use. All students will be running the reaction using 0.015 moles of their starting carbonyl compound and a slight excess of the Grignard reagent. You will form your Grignard reagent using 1.25 molar equivalents of the bromobenzcne (relative to the benzophenone) and 1.35 molar equivalents of magnesium. You will also need to determine the best way to measure out the reagents depending on whether they are solids of liquids at room temperature.
Required:
2. How many moles of Grignard reagent are you synthesizing? What mass of water could fully react with that amount of Grignard reagent? What volume of water would that be? 3. Step 11 of the procedure is called trituration and is used to extract any nonpolar impurities from your crude product (petroleum ether is a mixture of different isomers of pentanes, hexanes, and heptanes). Considering the possible side reactions that can occur, draw the structure of the likely non-polar side product that you are removing in this step?
RMX R R R M-X R Mg-X HO OH H R "R" R RBr RB RB 2 RBr Mg Mg Mg Mg RMgBr RMgBr RMB HO 0 CO R-R+MgB RH+HOMBr ROOMBr RCO MgBr benzophenone 1. PhMgBr 2. H'/HO OH tiphenylmethanol
Step by Step Solution
There are 3 Steps involved in it
Step: 1
2 The above figure shows the Grignard reagent and water reaction The metal ...
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
60952b64aa330_25616.pdf
180 KBs PDF File
60952b64aa330_25616.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


