Question
The literature values listed for the unknowns in this assignment are from either the Merck Index or the CRC Handbook, the two most used reference
The literature values listed for the unknowns in this assignment are from either the Merck Index or the CRC Handbook, the two most used reference handbooks. However, the values tend to vary slightly across literature sources and sometimes the temperatures are given as ranges. Give at least one reason for the variations in these reported temperatures.
In general, the boiling points of compounds increase down a group in the periodic table. The melting points and boiling points for the hydrogen compounds of group 6A elements are in the table below.
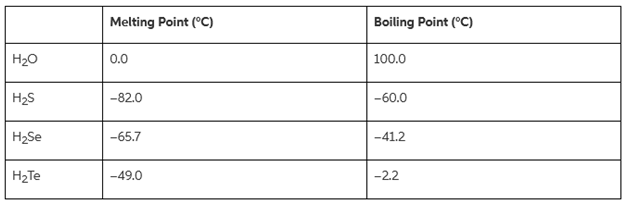
Use your understanding of chemistry to propose an explanation for the anomaly in the trend for the hydrogen compounds of group 6A elements shown in the table below.
HO HS HSe H Te Melting Point (C) 0.0 -82.0 -65.7 -49.0 Boiling Point (C) 100.0 -60.0 -41.2 -2.2
Step by Step Solution
3.40 Rating (159 Votes )
There are 3 Steps involved in it
Step: 1
In H2O there is hydrogen bonding which is strong inter molecular attraction force So H2...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


