Question
Wavelengths of spectral lines depend to some extent on the nuclear mass. This occurs because the nucleus is not an infinitely heavy stationary mass and
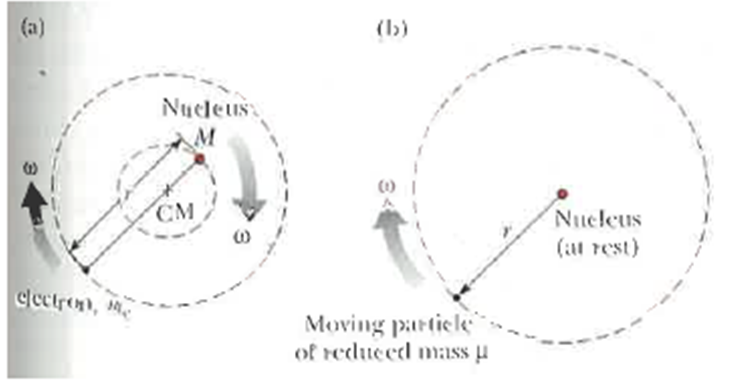
Wavelengths of spectral lines depend to some extent on the nuclear mass. This occurs because the nucleus is not an infinitely heavy stationary mass and both the electron and nucleus actually revolve around their common center of mass. It can be shown that a system of this type is entirely equivalent to a single particle of reduced mass M that revolves around the position of' the heavier particle at a distance equal to the electron?nucleus separation. See Figure P4.32. Here, ? = meM/(me + M), where me is the electron mass and M is the nuclear mass. To take the moving nucleus into account in the Bohr theory we replace me with ?. Thus Equation 4.30 becomes
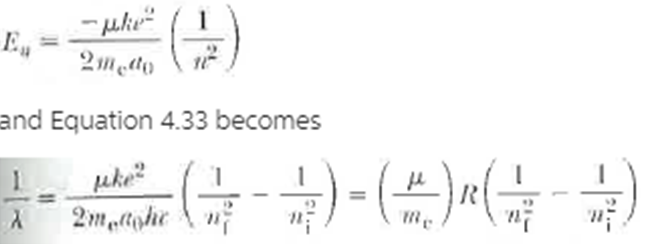
Determine the corrected values of wavelength for the first Balmer line (n = 3 to n = 2 transition) taking nuclear motion into account for (a) hydrogen, 1H. (b) deuterium, 2H. and (c) tritium. 3H. (Deuterium was actually discovered in 1932 by Harold Urey, who measured the small wavelength difference between 1H and 2H.) Figure P4.32 (a) Both the electron and the nucleus actually revolve around the center of mass (b) To calculate the effect of nuclear motion, the nucleus can be considered to be at rest and me is replaced by the reduced mass ?.
-poke 2m.do 11 and Equation 4.33 becomes (1) X G-7)-()G-3) 2metohen (a) electron, M Nucleus M CM 3 34 Moving particle of reduced mass Nucleus (at rest)
Step by Step Solution
3.46 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1
a Considering the reduced mass T...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


