Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Write the rate law for the following reaction assuming each reaction follows an elementary rate law. (1) CH, CH + H (2) CH +0,-CH-CH (3)
Write the rate law for the following reaction assuming each reaction follows an elementary rate law.
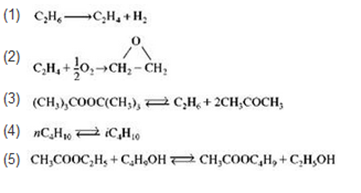
(1) CH, CH + H (2) CH +0,-CH-CH (3) (CH,),COOC(CH,), C,H; + 2CH,COCH, (4) nCH CH (5) CH,COOC;H,+C,H,OHTCH,COOC,H,+C,H,OH
Step by Step Solution
★★★★★
3.47 Rating (160 Votes )
There are 3 Steps involved in it
Step: 1
1 The provided elementary reaction is shown below Analysis of the above provided reaction clearly signifies that C 2 H 6 is reactant while C 2 H 4 and ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
60963040298e5_26691.pdf
180 KBs PDF File
60963040298e5_26691.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


