Question: a) A liquid is contained in a reactor vessel at 115 bar absolute pressure. It is transferred to a storage vessel through a 50
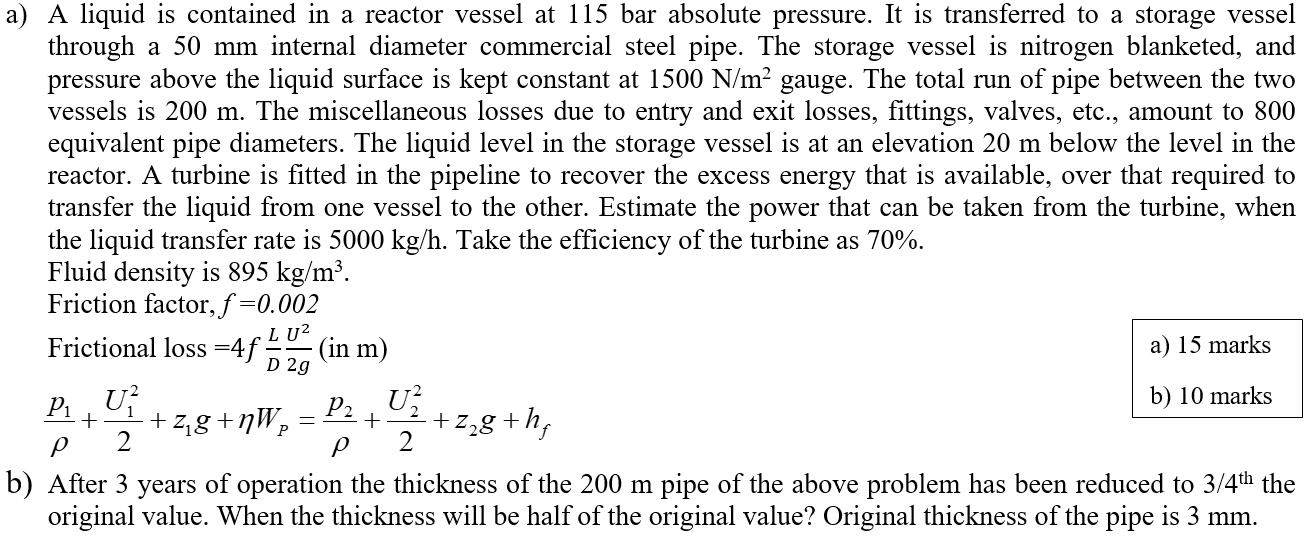
a) A liquid is contained in a reactor vessel at 115 bar absolute pressure. It is transferred to a storage vessel through a 50 mm internal diameter commercial steel pipe. The storage vessel is nitrogen blanketed, and pressure above the liquid surface is kept constant at 1500 N/m gauge. The total run of pipe between the two vessels is 200 m. The miscellaneous losses due to entry and exit losses, fittings, valves, etc., amount to 800 equivalent pipe diameters. The liquid level in the storage vessel is at an elevation 20 m below the level in the reactor. A turbine is fitted in the pipeline to recover the excess energy that is available, over that required to transfer the liquid from one vessel to the other. Estimate the power that can be taken from the turbine, when the liquid transfer rate is 5000 kg/h. Take the efficiency of the turbine as 70%. Fluid density is 895 kg/m. Friction factor, f=0.002 LU Frictional loss =4 =4f (in m) D 2g + +zg+nWp P_ U 2 b) After 3 years of operation the thickness of the 200 m pipe of the above problem has been reduced to 3/4th the original value. When the thickness will be half of the original value? Original thickness of the pipe is 3 mm. P U + +z8+hf P 2 = a) 15 marks b) 10 marks
Step by Step Solution
3.37 Rating (153 Votes )
There are 3 Steps involved in it
To solve the given problem we need to analyze both parts A and B a Power from the Turbine Given Flui... View full answer

Get step-by-step solutions from verified subject matter experts


