Answered step by step
Verified Expert Solution
Question
1 Approved Answer
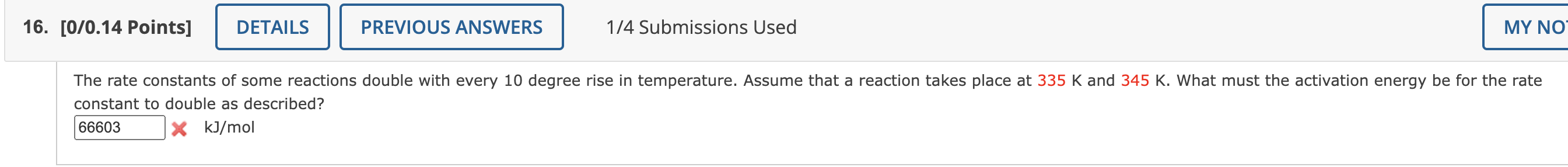
[0/0.14 Points] 1/4 Submissions Used The rate constants of some reactions double with every 10 degree rise in temperature. Assume that a reaction takes place
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


