Answered step by step
Verified Expert Solution
Question
1 Approved Answer
02. The global rates of SO2 oxidation have been measured with a platinum catalyst impregnated on the outer surface of 8181-in. cylindrical pellets of Al2O3.
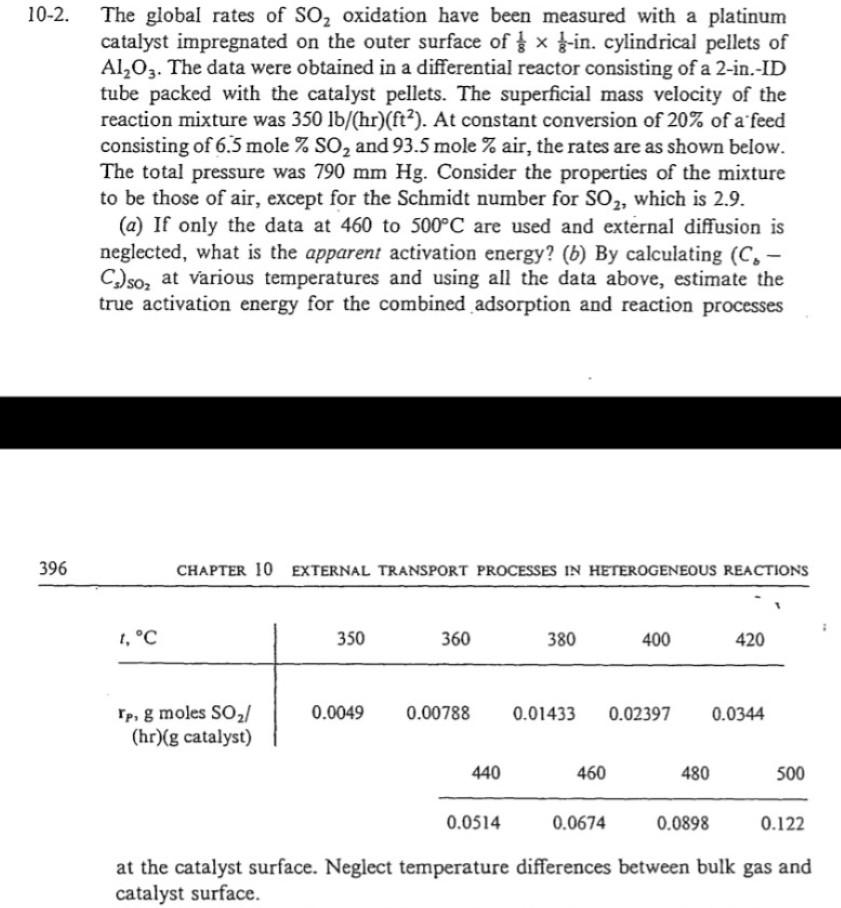
02. The global rates of SO2 oxidation have been measured with a platinum catalyst impregnated on the outer surface of 8181-in. cylindrical pellets of Al2O3. The data were obtained in a differential reactor consisting of a 2-in.-ID tube packed with the catalyst pellets. The superficial mass velocity of the reaction mixture was 350lb/(hr)(ft2). At constant conversion of 20% of a feed consisting of 6.5 mole %SO2 and 93.5 mole \% air, the rates are as shown below. The total pressure was 790mmHg. Consider the properties of the mixture to be those of air, except for the Schmidt number for SO2, which is 2.9. (a) If only the data at 460 to 500C are used and external diffusion is neglected, what is the apparent activation energy? (b) By calculating (Cb Cs)SO2 at various temperatures and using all the data above, estimate the true activation energy for the combined adsorption and reaction processes 396 CHAPTER 10 EXTERNAL TRANSPORT PROCESSES IN HETEROGENEOUS REACTIONS at the catalyst surface. Neglect temperature differences between bulk gas and catalyst surface
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


