Question
A chemist would like to monitor the progress of a chemical reaction using UV/V is spectroscopy. The spectrum for the starting material (solid line) and
A chemist would like to monitor the progress of a chemical reaction using UV/V is spectroscopy. The spectrum for the starting material (solid line) and the product (dashed line) are given. At what wavelength should the chemist set the detector to monitor the course of the reaction? Explain your answer.
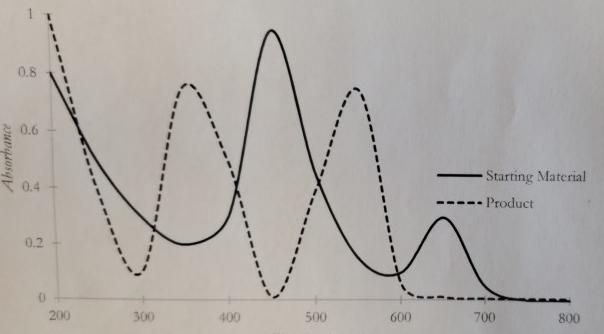
Absorbance 0.8 0.6 0.4 0.2 200 voll 300 400 500 600 Starting Material Product 700 800
Step by Step Solution
3.51 Rating (158 Votes )
There are 3 Steps involved in it
Step: 1
One of the most important factors affecting the wavelength of absorption by a molecule is the extent ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Biochemistry
Authors: Mary K. Campbell, Shawn O. Farrell
8th edition
9781305176621, 1285429109, 1305176626, 978-1285429106
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



