Question
0.85 mole/m. Determine the time when this concentration exceeds F CAI V V CA2 FIGURE 3.6 Two CSTRs in series. Information. The two systems
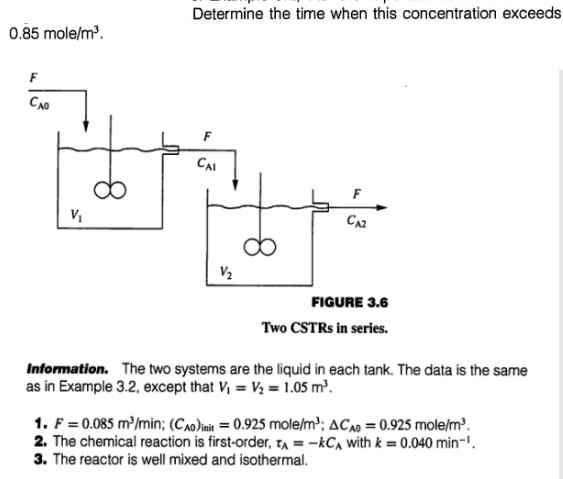
0.85 mole/m. Determine the time when this concentration exceeds F CAI V V CA2 FIGURE 3.6 Two CSTRs in series. Information. The two systems are the liquid in each tank. The data is the same as in Example 3.2, except that V = V = 1.05 m. 1. F= 0.085 m/min; (CAO) init = 0.925 mole/m: ACAD = 0.925 mole/m. 2. The chemical reaction is first-order, TA =-kCA with k=0.040 min-'. 3. The reactor is well mixed and isothermal.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
We can leverage the idea of a firstorder reaction in a Continuous Stirred Tank Reactor CSTR to tackl...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elements Of Chemical Reaction Engineering
Authors: H. Fogler
6th Edition
013548622X, 978-0135486221
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



