Answered step by step
Verified Expert Solution
Question
1 Approved Answer
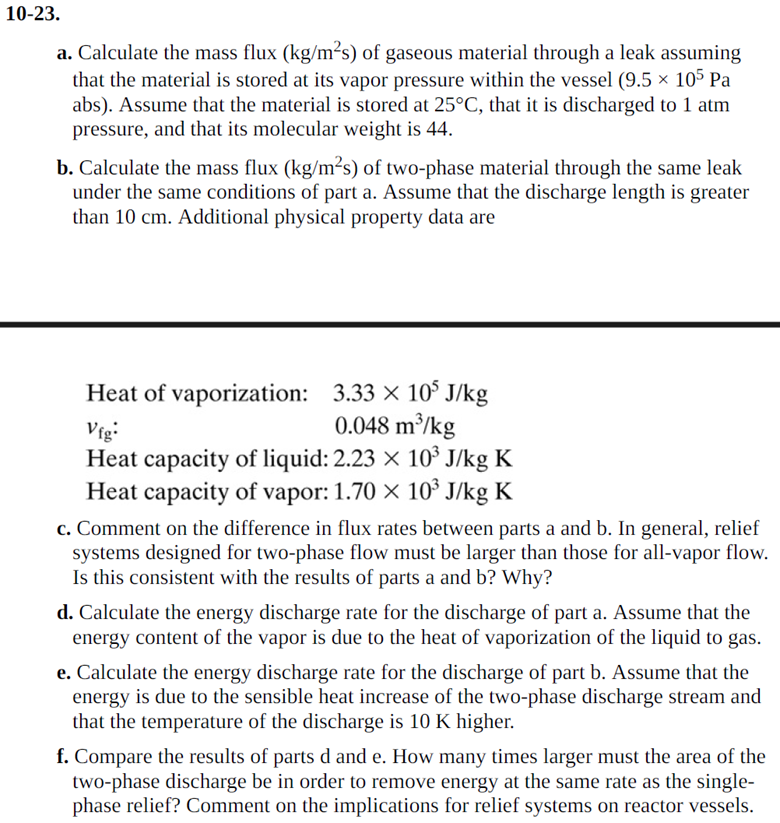
1 0 - 2 3 . a . Calculate the mass flux ( k g m 2 ( s ) ) of gaseous material through
a Calculate the mass flux of gaseous material through a leak assuming
that the material is stored at its vapor pressure within the vessel
abs Assume that the material is stored at that it is discharged to atm
pressure, and that its molecular weight is
b Calculate the mass flux of twophase material through the same leak
under the same conditions of part a Assume that the discharge length is greater
than Additional physical property data are
Heat vaporization:
:
Heat capacity liquid:
Heat capacity vapor:
c Comment on the difference in flux rates between parts a and b In general, relief
systems designed for twophase flow must be larger than those for allvapor flow.
Is this consistent with the results of parts a and Why?
d Calculate the energy discharge rate for the discharge of part a Assume that the
energy content of the vapor is due to the heat of vaporization of the liquid to gas.
e Calculate the energy discharge rate for the discharge of part b Assume that the
energy is due to the sensible heat increase of the twophase discharge stream and
that the temperature of the discharge is higher.
f Compare the results of parts and How many times larger must the area of the
twophase discharge be in order to remove energy at the same rate as the single
phase relief? Comment on the implications for relief systems on reactor vessels.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


