Answered step by step
Verified Expert Solution
Question
1 Approved Answer
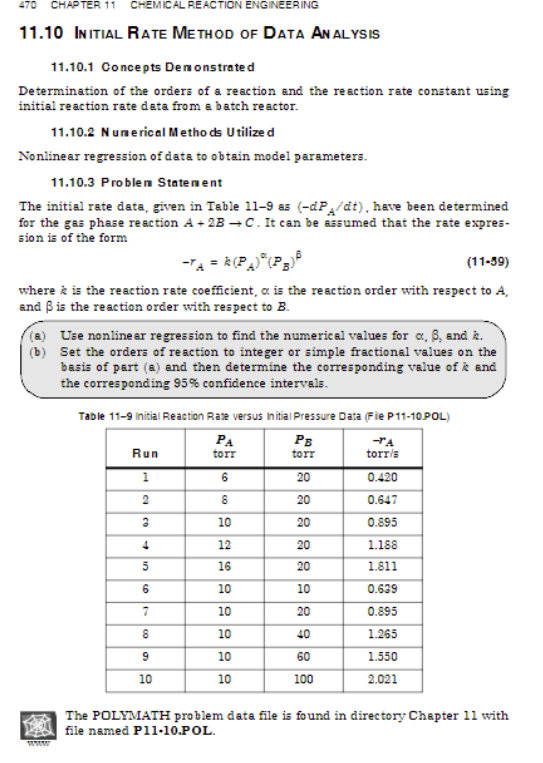
1 1 . 1 0 In itial Rate Method of Data Analysis 1 1 . 1 0 . 1 Concepts Denonstrated Determination of the orders
In itial Rate Method of Data Analysis
Concepts Denonstrated
Determination of the orders of a reaction and the reaction rate conatant using
initial reaction rate data from a batch reactor.
N une rical Methods U tilize d
Nonlinear regression of data to obtain model parameters.
Problen Statenent
The initial rate data, given in Table as have been determined
for the gas phase reaction It can be assumed that the rate expres
sion is of the form
where is the reaction rate coefficient, is the reaction order with respect to
and is the reaction order with respect to
a Use nonline ar regression to find the numerical values for and
b Set the orders of reaction to integer or simple fractional values on the
basis of part a and then determine the corresponding value of and
the corresponding confidence intervals.
Table Initial Reaction Rave versus Initial Pressure Data Fie P POL
The POLYMATH problem data file is found in directory Chapter with
file named PPOL.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


