Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1 ) 1 . 2 5 kmol of C 2 H 4 per kmol of C l 2 are fed to the reactor. As before,
kmol of per kmol of are fed to the reactor. As before, ethene and chlorine react together to form dichloroethane via the main reaction and some of the ethene and chlorine also react to form tricholorethane via a side reaction However, a recycle for unreacted ethene is now being introduced. In this set up the byproducts leave with the dichloroethane. The singlepass conversion of chlorine is still and the selectivity of over is still The plant is to be designed to produce tey of dichloroethane and is to operate for per year.
aCalculate the feed rates of ethene and chlorine required if the unreacted ethene is recycled.
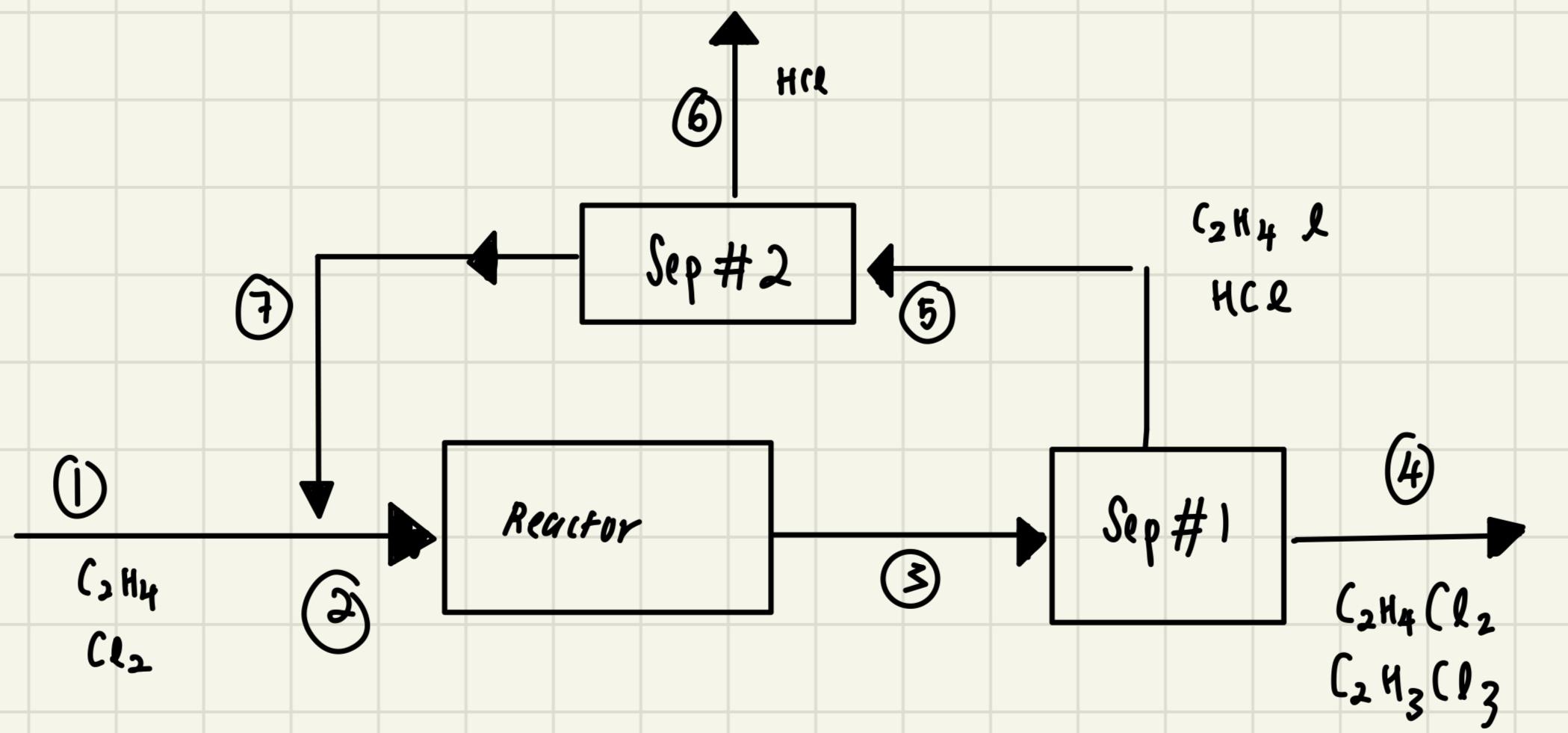
Part The separation stages from the process of Q are to be redesigned. Now, the and the are separated first in unit Sep # and leave as a mixed product stream All of the remaining gases, and are recycled through an absorption unit Sep # which allows for recyle of any unreacted ethene and selectively removes a fraction of the The production rate of dichloroethane is to be maintained at tey and the plant still operates for Calc
ulate the steadystate flowrates of in the recycle before the absorber in for the cases where
a All of the in the stream is removed in the Sep # absorber
b of the in stream is removed in the Sep # absorber the unreacted ethene is recycled.related to the image given
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


