Question
1. 1 kg aluminum dumbbell initially held 2 meters above the floor is allowed to fall. This experiment is performed inside a room whose dimensions
1. 1 kg aluminum dumbbell initially held 2 meters above the floor is allowed to fall. This experiment is performed inside a room whose dimensions are 10m ́ 5m ́ 3m. Assuming that the walls of the room do not conduct heat and that the room is empty (no other objects are present besides the dumbbell itself). Estimate the change of entropy of the system (defined as the room + the dumbbell) as a result of the process.
2. Recall the (thought) experiment described reversible vs. irreversible processes. We had 1 mole of a thermally insulated ideal gas in a cylinder under a piston of mass ????! (in vacuum). Then a weight with a mass ???? " − ????! was first placed on top of the piston and - after equilibrium was reached - it was removed. We have then calculated the final temperature of the gas.
A. What is the change of the entropy Δ???? of the gas as a result of this process (adding and removing the weight)? The molar heat capacity ????̅# of the gas is known and is temperature independent. Is your result consistent with the 2nd law of thermodynamics?
B. What is the change of the gas entropy when the experiment is modified such that the mass ???? " − ????! comes in the form of a pile of sand and is gradually (and very slowly) added to the piston and then removed in the same fashion?
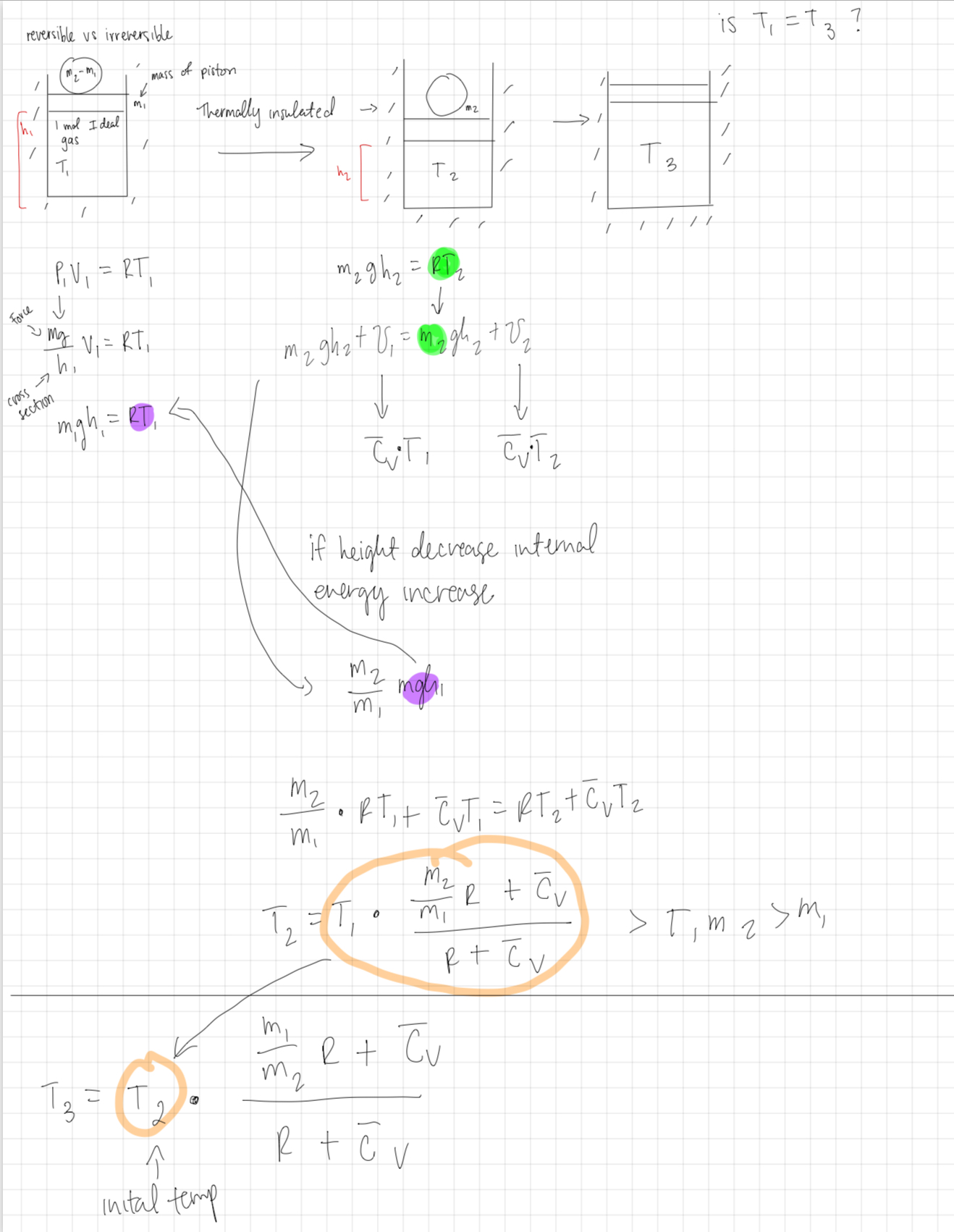
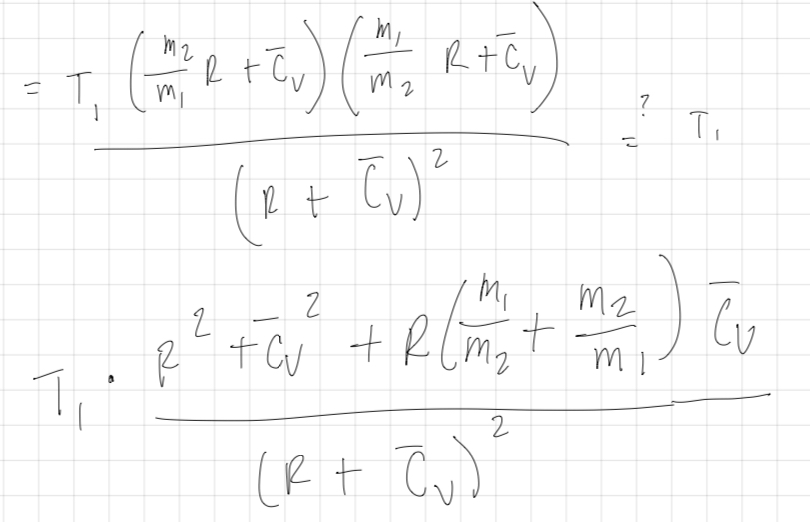

hi / reversible vs irreversible -m mass of I mol I deal gas T 1 piston Thermally insulated -> m T = RT 2 Force PV = RT Umg Cross section h V = RT mgh = 1 m 2ghz gh + V m2 cutz T 2 inital temp if height decrease internal energy increase M2 M 12 m m 2 0 M2 m mghi RT, + CUT, = RT+ Cv Iz T. 0 M2 R + Cv Mi R + Cv R + C v R + C v T 3 /// is T, = T ? >T,mz>m,
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


