Question
1. 120g of an organic compound that contains only carbon and hydrogen gives 330g of CO2 and 270g of water on complete combustion. The
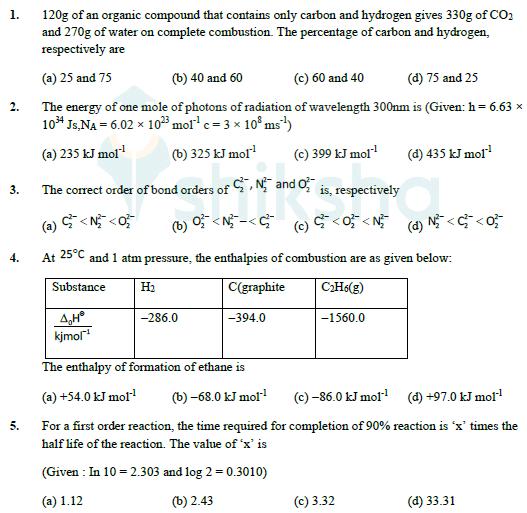
1. 120g of an organic compound that contains only carbon and hydrogen gives 330g of CO2 and 270g of water on complete combustion. The percentage of carbon and hydrogen, respectively are (a) 25 and 75 2. (b) 40 and 60 (c) 60 and 40 (d) 75 and 25 The energy of one mole of photons of radiation of wavelength 300nm is (Given: h = 6.63 1034 Js,NA = 6.02 1023 mol c = 3 10 ms) (a) 235 kJ mol 3. The correct order of bond orders of (b) 325 kJ mol CN and O (c) 399 kJ mol (d) 435 kJ mol is, respectively (a) <
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemistry The Central Science
Authors: Theodore Brown, Eugene LeMay, Bruce Bursten, Catherine Murphy, Patrick Woodward
12th edition
321696727, 978-0132175081, 978-0321696724
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



