Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. [15 marks total] Consider a mass of air in the atmosphere (a cold front), consisting of a rectangular prism 100. km x 100.
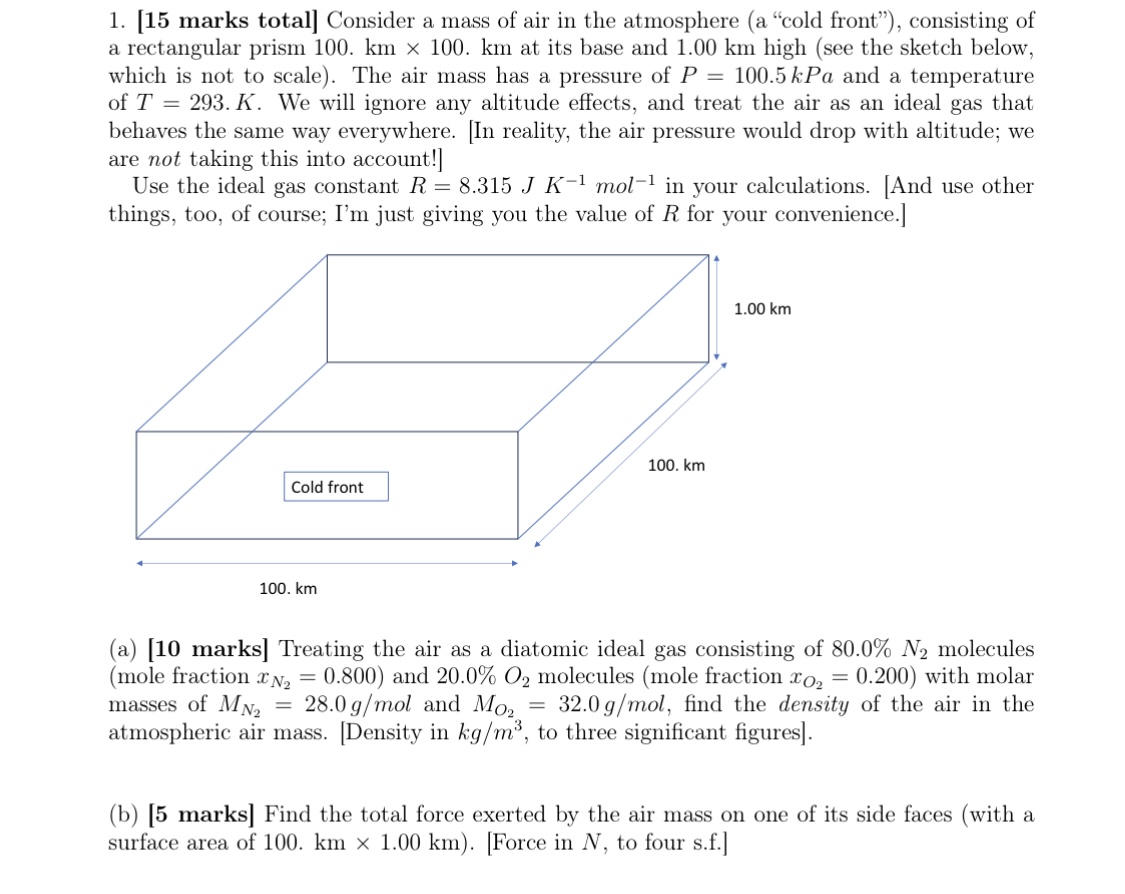
1. [15 marks total] Consider a mass of air in the atmosphere (a "cold front"), consisting of a rectangular prism 100. km x 100. km at its base and 1.00 km high (see the sketch below, which is not to scale). The air mass has a pressure of P = 100.5 kPa and a temperature of T = 293. K. We will ignore any altitude effects, and treat the air as an ideal gas that behaves the same way everywhere. [In reality, the air pressure would drop with altitude; we are not taking this into account!] Use the ideal gas constant R = 8.315 J K-1 mol-1 in your calculations. [And use other things, too, of course; I'm just giving you the value of R for your convenience.] 100. km Cold front 100. km 1.00 km (a) [10 marks] Treating the air as a diatomic ideal gas consisting of 80.0% N2 molecules (mole fraction x N = 0.800) and 20.0% O2 molecules (mole fraction xo = 0.200) with molar 28.0 g/mol and Moz 32.0 g/mol, find the density of the air in the = = masses of MN2 atmospheric air mass. [Density in kg/m, to three significant figures]. (b) [5 marks] Find the total force exerted by the air mass on one of its side faces (with a surface area of 100. km x 1.00 km). [Force in N, to four s.f.]
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


