Question
1. 25 cm of iron (II) sulphate reacts with 24.3 cm3 of 0.02M potassium manganate (VII) solution. Calculate the concentration of the iron( sulphate)
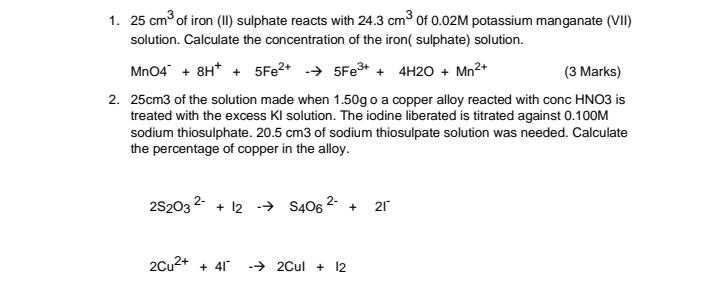
1. 25 cm of iron (II) sulphate reacts with 24.3 cm3 of 0.02M potassium manganate (VII) solution. Calculate the concentration of the iron( sulphate) solution. Mn04 + 8H* + 5FE2+ - 5FE3+ + 4H20 + Mn2+ (3 Marks) 2. 25cm3 of the solution made when 1.50g o a copper alloy reacted with conc HNO3 is treated with the excess KI solution. The iodine liberated is titrated against 0.10OM sodium thiosulphate. 20.5 cm3 of sodium thiosulpate solution was needed. Calculate the percentage of copper in the alloy. 2S203 2 + 12 - S406 2- 21 + 2Cu2+ + 41 - 2Cul + 12
Step by Step Solution
3.36 Rating (149 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
General Chemistry
Authors: Darrell Ebbing, Steven D. Gammon
9th edition
978-0618857487, 618857486, 143904399X , 978-1439043998
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



