Question: 1. (28 points total) The sketch below shows an PV isotherm for an ideal gas with the locations of two states, State 1 (at P1,
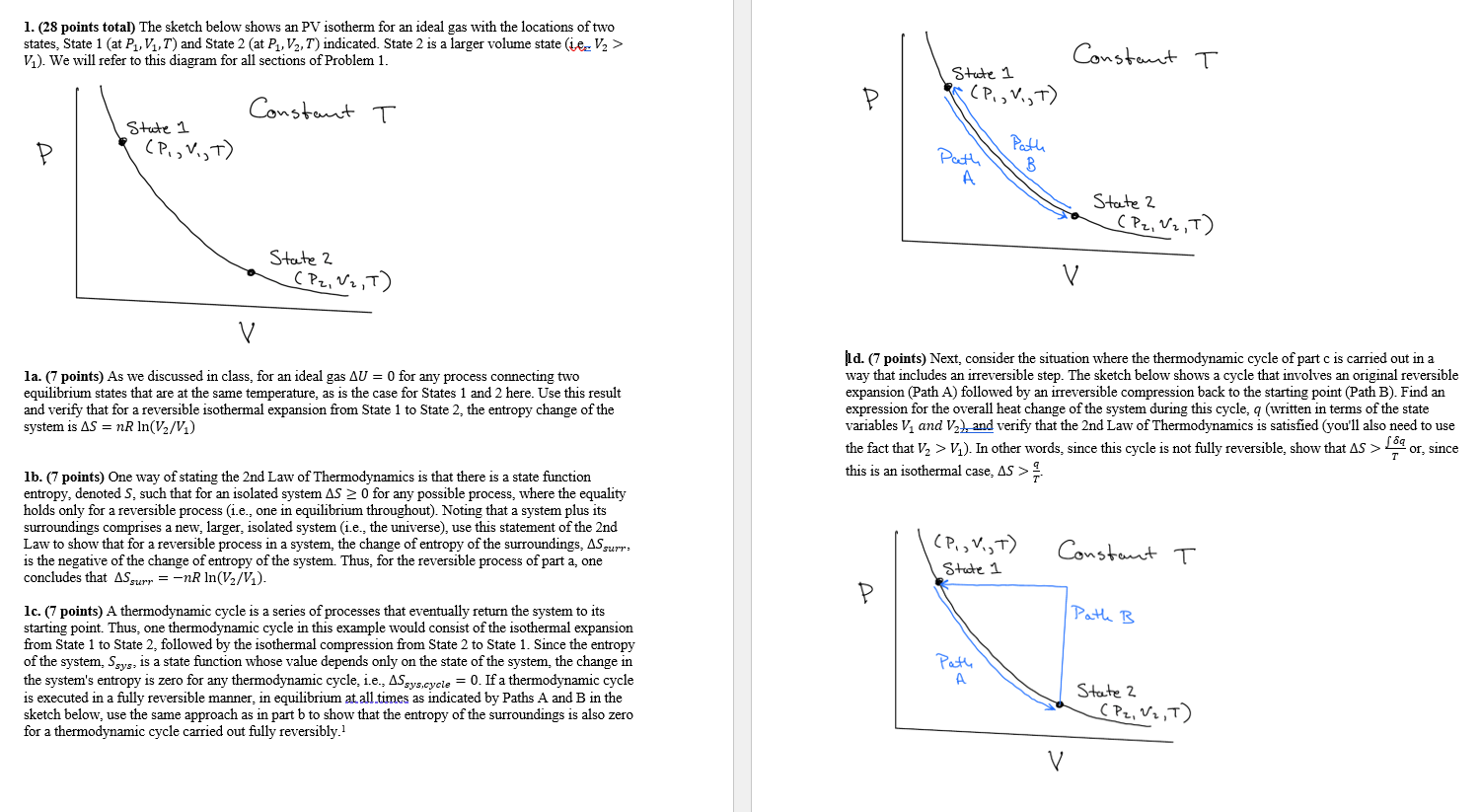
1. (28 points total) The sketch below shows an PV isotherm for an ideal gas with the locations of two states, State 1 (at P1, V1,T) and State 2 (at P1, V2,7) indicated. State 2 is a larger volume state (ie V2 > V.). We will refer to this diagram for all sections of Problem 1. Constant T State 1 Constant T (P., V,T) P State 1 (P., V.,T) Path . Path B State 2 (P2, V2,T) State 2 (P2, W2,T) V v la. (7 points) As we discussed in class, for an ideal gas AU = 0 for any process connecting two equilibrium states that are at the same temperature, as is the case for States 1 and 2 here. Use this result and verify that for a reversible isothermal expansion from State 1 to State 2, the entropy change of the system is AS = nR In(V2/V) 1 d. (7 points) Next, consider the situation where the thermodynamic cycle of part c is carried out in a way that includes an irreversible step. The sketch below shows a cycle involves an original reversible expansion (Path A) followed by an irreversible compression back to the starting point (Path B). Find an expression for the overall heat change of the system during this cycle, q (written in terms of the state variables V and V2), and verify that the 2nd Law of Thermodynamics is satisfied (you'll also need to use the fact that V, > V2). In other words, since this cycle is not fully reversible, show that AS > 189 or, since this is an isothermal case, AS > 1b. (7 points) One way of stating the 2nd Law of Thermodynamics is that there is a state function entropy, denoted S, such that for an isolated system AS 0 for any possible process, where the equality holds only for a reversible process (i.e., one in equilibrium throughout). Noting that a system plus its a surroundings comprises a new, larger, isolated system (i.e., the universe), use this statement of the 2nd Law to show that for a reversible process in a system, the change of entropy of the surroundings, ASgurr. is the negative of the change of entropy of the system. Thus, for the reversible process of part a, one concludes that ASgurr = -NR In(V2/V1). CPV,T) State 1 Constant T Path B lc. (7 points) A thermodynamic cycle is a series of processes that eventually return the system to its starting point. Thus, one thermodynamic cycle in this example would consist of the isothermal expansion from State 1 to State 2, followed by the isothermal compression from State 2 to State 1. Since the entropy of the system, Ssys, is a state function whose value depends only on the state of the system, the change in the system's entropy is zero for any thermodynamic cycle, i.e., ASsys.cycle = 0. If a thermodynamic cycle is executed in a fully reversible manner, in equilibrium at all times as indicated by Paths A and B in the sketch below, use the same approach as in part b to show that the entropy of the surroundings is also zero for a thermodynamic cycle carried out fully reversibly Path A State 2 (P2, V2,T) V 1. (28 points total) The sketch below shows an PV isotherm for an ideal gas with the locations of two states, State 1 (at P1, V1,T) and State 2 (at P1, V2,7) indicated. State 2 is a larger volume state (ie V2 > V.). We will refer to this diagram for all sections of Problem 1. Constant T State 1 Constant T (P., V,T) P State 1 (P., V.,T) Path . Path B State 2 (P2, V2,T) State 2 (P2, W2,T) V v la. (7 points) As we discussed in class, for an ideal gas AU = 0 for any process connecting two equilibrium states that are at the same temperature, as is the case for States 1 and 2 here. Use this result and verify that for a reversible isothermal expansion from State 1 to State 2, the entropy change of the system is AS = nR In(V2/V) 1 d. (7 points) Next, consider the situation where the thermodynamic cycle of part c is carried out in a way that includes an irreversible step. The sketch below shows a cycle involves an original reversible expansion (Path A) followed by an irreversible compression back to the starting point (Path B). Find an expression for the overall heat change of the system during this cycle, q (written in terms of the state variables V and V2), and verify that the 2nd Law of Thermodynamics is satisfied (you'll also need to use the fact that V, > V2). In other words, since this cycle is not fully reversible, show that AS > 189 or, since this is an isothermal case, AS > 1b. (7 points) One way of stating the 2nd Law of Thermodynamics is that there is a state function entropy, denoted S, such that for an isolated system AS 0 for any possible process, where the equality holds only for a reversible process (i.e., one in equilibrium throughout). Noting that a system plus its a surroundings comprises a new, larger, isolated system (i.e., the universe), use this statement of the 2nd Law to show that for a reversible process in a system, the change of entropy of the surroundings, ASgurr. is the negative of the change of entropy of the system. Thus, for the reversible process of part a, one concludes that ASgurr = -NR In(V2/V1). CPV,T) State 1 Constant T Path B lc. (7 points) A thermodynamic cycle is a series of processes that eventually return the system to its starting point. Thus, one thermodynamic cycle in this example would consist of the isothermal expansion from State 1 to State 2, followed by the isothermal compression from State 2 to State 1. Since the entropy of the system, Ssys, is a state function whose value depends only on the state of the system, the change in the system's entropy is zero for any thermodynamic cycle, i.e., ASsys.cycle = 0. If a thermodynamic cycle is executed in a fully reversible manner, in equilibrium at all times as indicated by Paths A and B in the sketch below, use the same approach as in part b to show that the entropy of the surroundings is also zero for a thermodynamic cycle carried out fully reversibly Path A State 2 (P2, V2,T) V
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


