Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1) (30 pts) Nitrogen (N) flows through a pipe, entering at at 4 m/sec at 1000 kPa, 207C. For a pipe inside diameter of
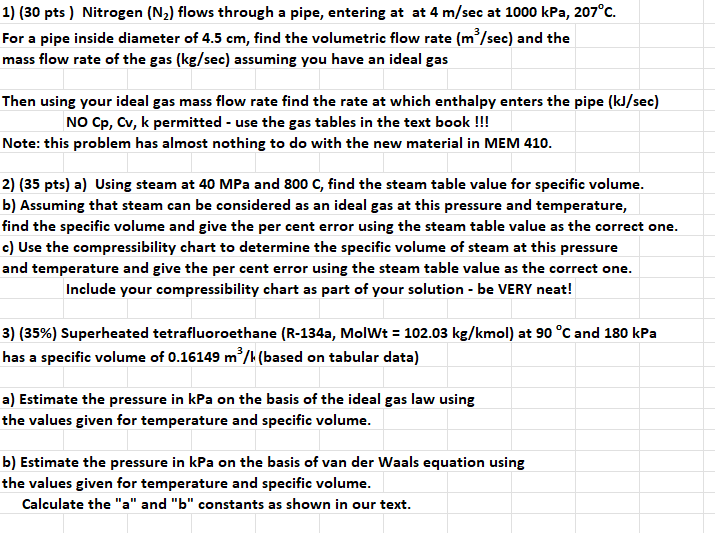
1) (30 pts) Nitrogen (N) flows through a pipe, entering at at 4 m/sec at 1000 kPa, 207C. For a pipe inside diameter of 4.5 cm, find the volumetric flow rate (m/sec) and the mass flow rate of the gas (kg/sec) assuming you have an ideal gas Then using your ideal gas mass flow rate find the rate at which enthalpy enters the pipe (kJ/sec) NO Cp, Cv, k permitted - use the gas tables in the text book !!! Note: this problem has almost nothing to do with the new material in MEM 410. 2) (35 pts) a) Using steam at 40 MPa and 800 C, find the steam table value for specific volume. b) Assuming that steam can be considered as an ideal gas at this pressure and temperature, find the specific volume and give the per cent error using the steam table value as the correct one. c) Use the compressibility chart to determine the specific volume of steam at this pressure and temperature and give the per cent error using the steam table value as the correct one. Include your compressibility chart as part of your solution - be VERY neat! 3) (35%) Superheated tetrafluoroethane (R-134a, MolWt = 102.03 kg/kmol) at 90 C and 180 kPa has a specific volume of 0.16149 m/h (based on tabular data) a) Estimate the pressure in kPa on the basis of the ideal gas law using the values given for temperature and specific volume. b) Estimate the pressure in kPa on the basis of van der Waals equation using the values given for temperature and specific volume. Calculate the "a" and "b" constants as shown in our text.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


