Answered step by step
Verified Expert Solution
Question
1 Approved Answer
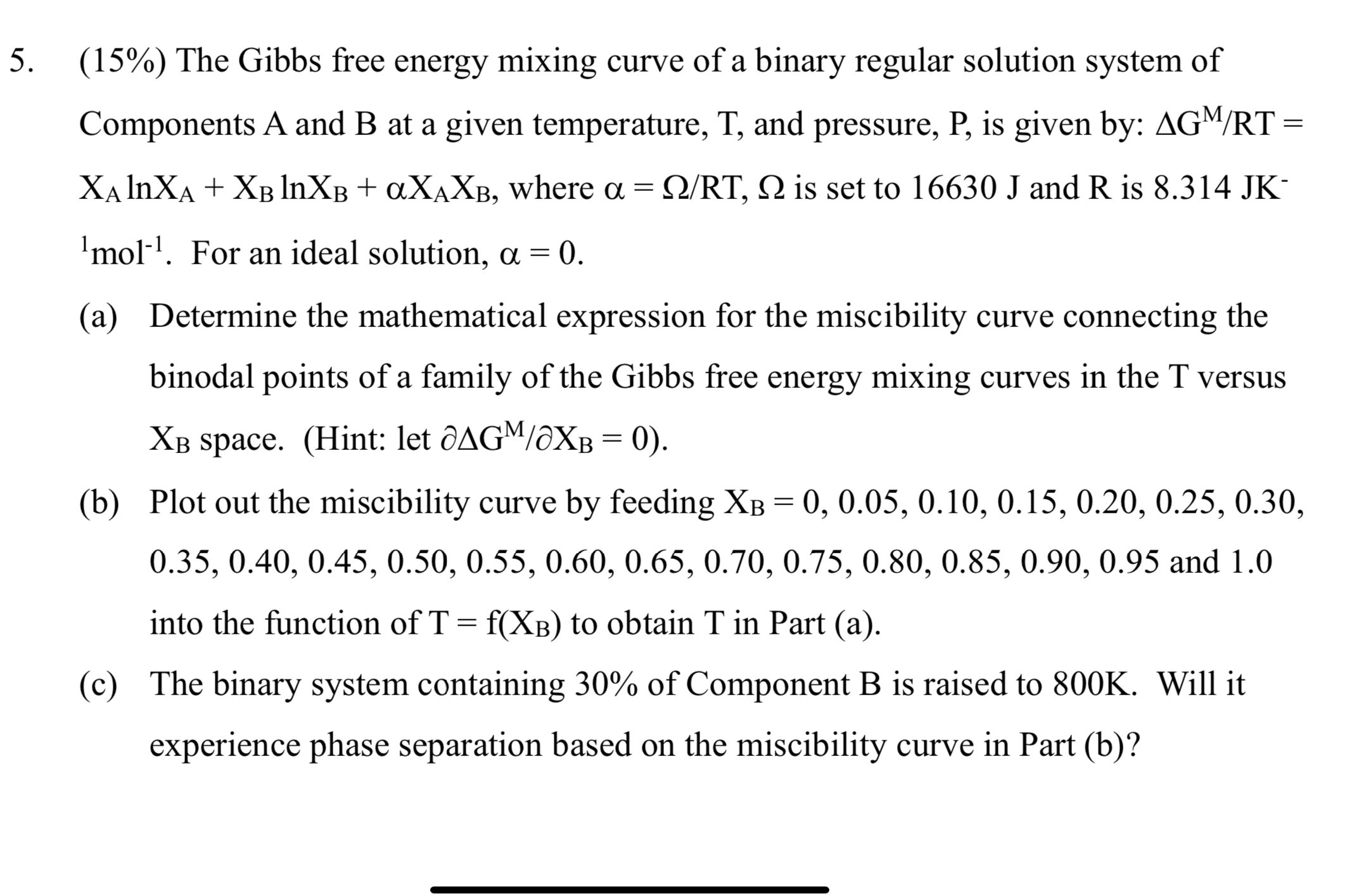
( 1 5 % ) The Gibbs free energy mixing curve of a binary regular solution system of Components A and B at a given
The Gibbs free energy mixing curve of a binary regular solution system of Components A and at a given temperature, and pressure, is given by: where is set to and is For an ideal solution,
a Determine the mathematical expression for the miscibility curve connecting the binodal points of a family of the Gibbs free energy mixing curves in the versus space. Hint: let del
b Plot out the miscibility curve by feeding and into the function of to obtain in Part a
c The binary system containing of Component B is raised to Will it experience phase separation based on the miscibility curve in Part b
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


