Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. (50p) 100kmol mixture containing 35% ethanol (A) and 65% water (B) by mole; It is heated and evaporated under differential conditions at a pressure
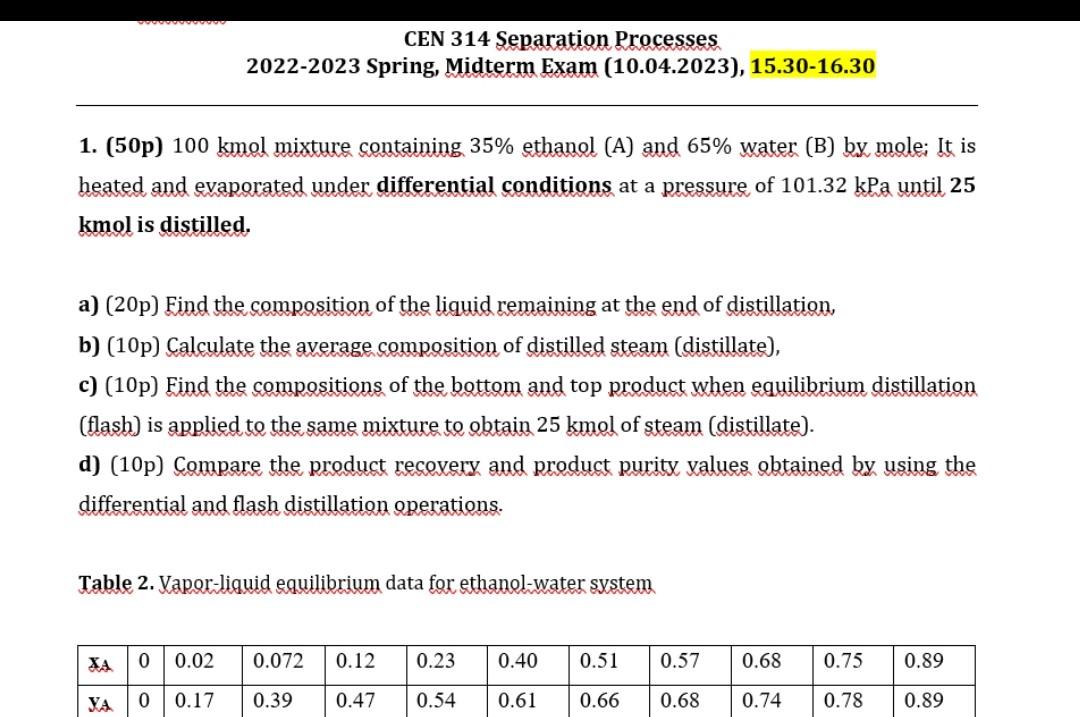
1. (50p) 100kmol mixture containing 35% ethanol (A) and 65% water (B) by mole; It is heated and evaporated under differential conditions at a pressure of 101.32kPa until 25 kmol is distilled. a) (20p) Find the composition of the liquid remaining at the end of distillation, b) (10p) Calculate the average composition of distilled steam (distillate), c) (10p) Find the compositions of the bottom and top product when equilibrium distillation (flash) is applied to the same mixture to obtain 25kmol of steam (distillate). d) (10p) Compare the product recevery and product purity values obtained by using the differential and flash distillation operations. Table 2. Vapor-liquid equilibrium data for ethanol-water system
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


