Answered step by step
Verified Expert Solution
Question
1 Approved Answer
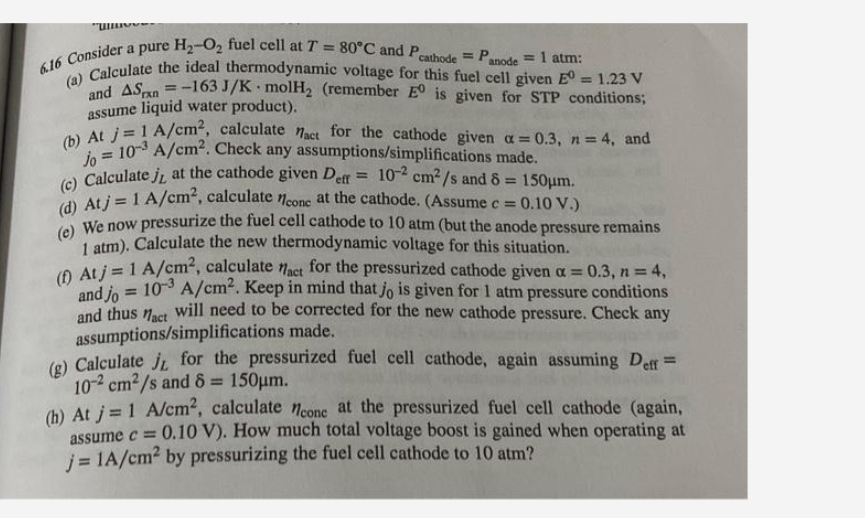
1 6 Consider a pure H 2 - O 2 fuel cell at T = 8 0 C and P c a t h o
Consider a pure fuel cell at and atm :
a Calculate the ideal thermodynamic voltage for this fuel cell given and remember is given for STP conditions; assume liquid water product
b At calculate for the cathode given and Check any assumptionssimplifications made.
c Calculate at the cathode given and
d At calculate at the cathode. Assume
c We now pressurize the fuel cell cathode to atm but the anode pressure remains atm. Calculate the new thermodynamic voltage for this situation.
f Atj calculate for the pressurized cathode given and Keep in mind that is given for atm pressure conditions and thus will need to be corrected for the new cathode pressure. Check any assumptionssimplifications made.
g Calculate for the pressurized fuel cell cathode, again assuming and
b At calculate at the pressurized fuel cell cathode again assume How much total voltage boost is gained when operating at by pressurizing the fuel cell cathode to atm
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


