Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. A 1.065g sample of stainless steel was dissolved in HCI (this process convert Cr to Cr3+ and Fe toFe+3), and diluted to 500.0 ml.
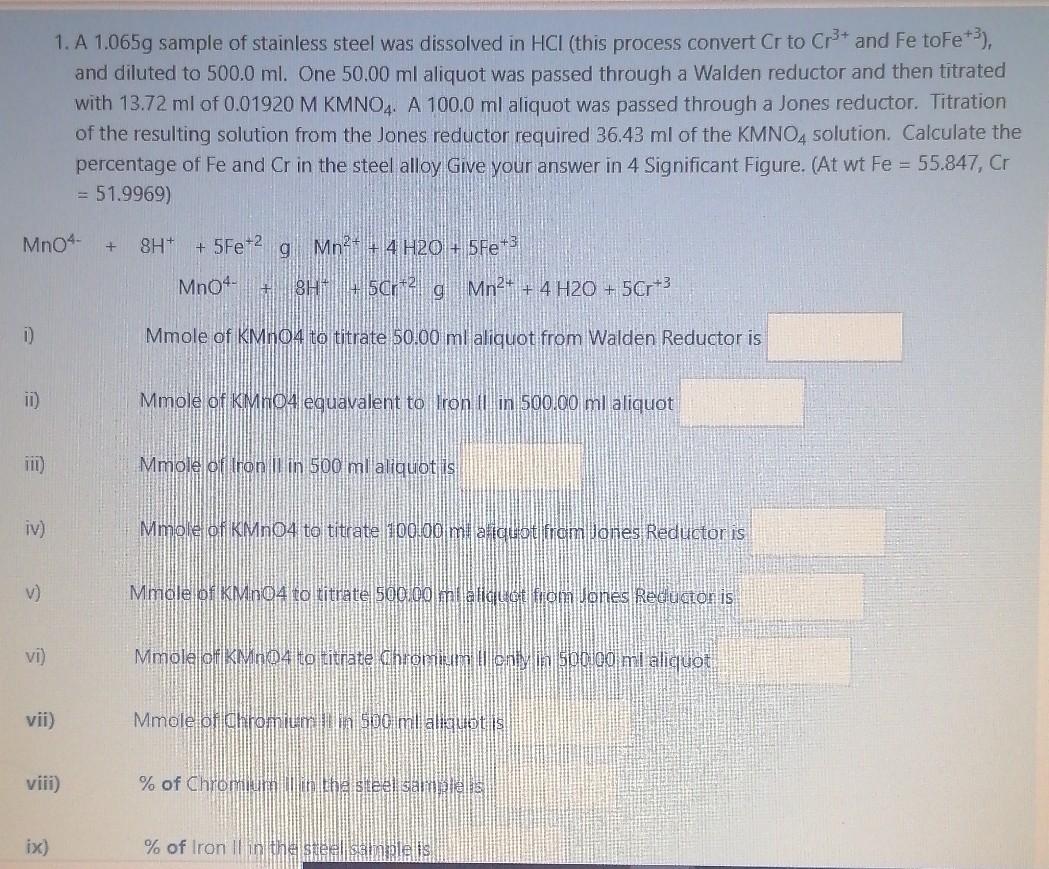
1. A 1.065g sample of stainless steel was dissolved in HCI (this process convert Cr to Cr3+ and Fe toFe+3), and diluted to 500.0 ml. One 50.00 ml aliquot was passed through a Walden reductor and then titrated with 13.72 ml of 0.01920 M KMNO4. A 100.0 ml aliquot was passed through a Jones reductor. Titration of the resulting solution from the Jones reductor required 36.43 ml of the KMNO4 solution. Calculate the percentage of Fe and Cr in the steel alloy Give your answer in 4 Significant Figure. (At wt Fe = 55.847, Cr = 51.9969) Mn04 + 8H+ + 5Fe-2 g Mn2+ + 4 H20 + 5Fe+ MnO4- + 8H + 50r 21 Mn2+ + 4 H20 + 5Cr3 D Mmole of KMnO4 to titrate 50.00 ml aliquot from Walden Reductor is ii) Mmole of KMnO4 eguavalent to Iron in 500.00 ml aliquot 111) Mmole of tron I in 500 ml aliquot is iv) Mmole of KMnO4 to titrate 10000 mi aliquot from Jones Reductor is v) Mmole of KMnO4 to titrate 500.00 ml aliquide from Jones Reducton is vi) Mmole of KMnO4 to titnate dhromium tenmin 500.00 ml aliquot vii) Mmole of chromium Soo ml aliquot is viii) % of Chromium ch steel samelis ix) % of Iron Il in the sue
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


