Question
1. A 300.0 g metal block absorbs 3.47 103 J of heat to raise its temperature by 30.0 K. What is the specific heat
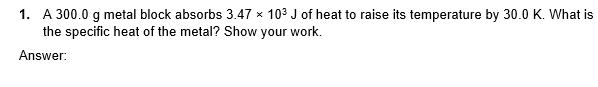
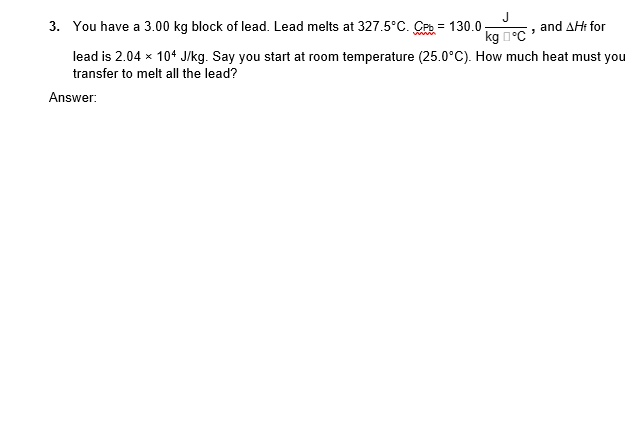
1. A 300.0 g metal block absorbs 3.47 103 J of heat to raise its temperature by 30.0 K. What is the specific heat of the metal? Show your work. Answer: 3. You have a 3.00 kg block of lead. Lead melts at 327.5C. CPb = 130.0 J kg, and AHt for lead is 2.04 x 104 J/kg. Say you start at room temperature (25.0C). How much heat must you transfer to melt all the lead? Answer:
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Physics
Authors: Alan Giambattista, Betty Richardson, Robert Richardson
2nd edition
77339681, 978-0077339685
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



