Question
1. (a) Calculate P. (Assume suitable values for the required parameters). Atmosphere AIR P=? h=15 mm (2 M) (b) An ideal gas with Cv=5/2
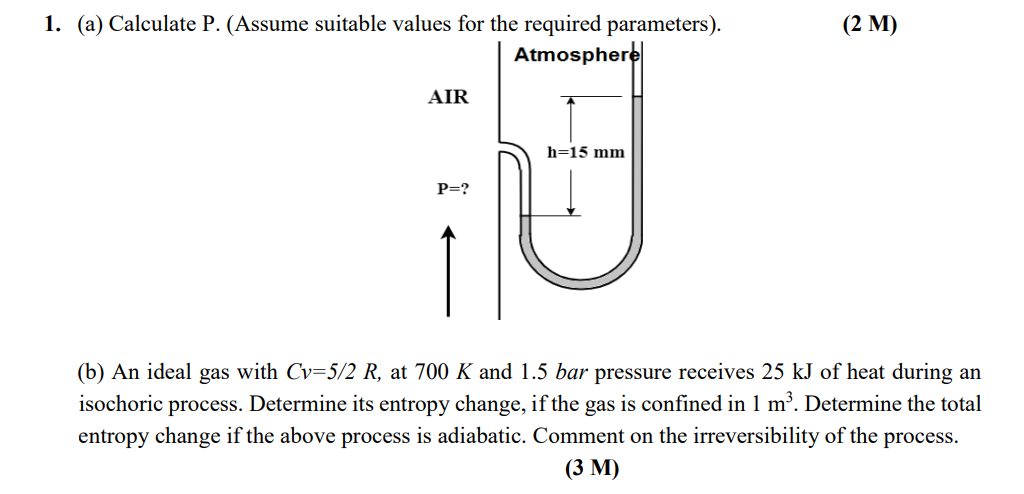
1. (a) Calculate P. (Assume suitable values for the required parameters). Atmosphere AIR P=? h=15 mm (2 M) (b) An ideal gas with Cv=5/2 R, at 700 K and 1.5 bar pressure receives 25 kJ of heat during an isochoric process. Determine its entropy change, if the gas is confined in 1 m. Determine the total entropy change if the above process is adiabatic. Comment on the irreversibility of the process. (3 M)
Step by Step Solution
There are 3 Steps involved in it
Step: 1
This problem is a twopart question that pertains to thermodynamics First I will address part a To calculate the pressure P at the open end of the tube we can make use of the pressure difference create...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemical Biochemical And Engineering Thermodynamics
Authors: Stanley I. Sandler
5th Edition
047050479X, 978-0470504796
Students also viewed these Mechanical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



