Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1- (a) Copper crystallizes in fcc structure (atomic mass of copper, 63.54 u). If the atom radius of copper is 0.1278 nm, calculate: (a)
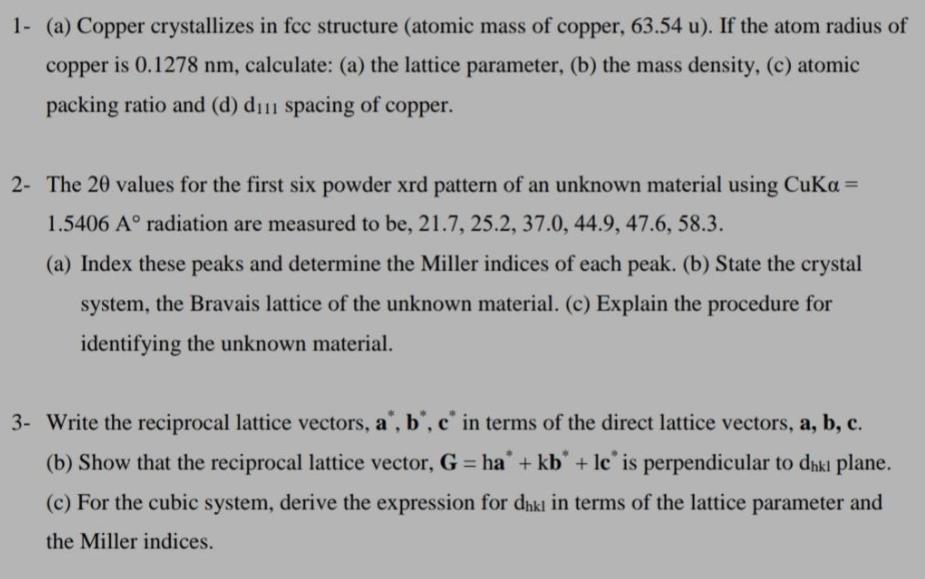
1- (a) Copper crystallizes in fcc structure (atomic mass of copper, 63.54 u). If the atom radius of copper is 0.1278 nm, calculate: (a) the lattice parameter, (b) the mass density, (c) atomic packing ratio and (d) di spacing of copper. 2- The 20 values for the first six powder xrd pattern of an unknown material using Cuka = 1.5406 A radiation are measured to be, 21.7, 25.2, 37.0, 44.9, 47.6, 58.3. (a) Index these peaks and determine the Miller indices of each peak. (b) State the crystal system, the Bravais lattice of the unknown material. (c) Explain the procedure for identifying the unknown material. 3- Write the reciprocal lattice vectors, a, b, c in terms of the direct lattice vectors, a, b, c. (b) Show that the reciprocal lattice vector, G = ha + kb + lc is perpendicular to dki plane. (c) For the cubic system, derive the expression for dekl in terms of the lattice parameter and the Miller indices.
Step by Step Solution
★★★★★
3.30 Rating (159 Votes )
There are 3 Steps involved in it
Step: 1
1 a To calculate the lattice parameter a of copper in the facecentered cubic fcc structure we can use the relationship between the atomic radius r and the lattice parameter a in an fcc structure a 4 r ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


